 | ||
Helium is the most unreactive element, so it was commonly believed that helium compounds do not exist at all. Helium's first ionization energy of 24.57 eV is the highest of any element. Helium has a complete shell of electrons, and in this form the atom does not readily accept any extra electrons or join with anything to make covalent compounds. The electron affinity is 0.080 eV, which is very close to zero. The helium atom is small with the radius of the outer electron shell at 0.29 Å. The atom is very hard with a Pearson's hardness of 12.3 eV. It has the lowest polarizability of any kind of atom. However very weak van der Waals forces exist between helium and other atoms. This force may exceed repulsive forces. So at extremely low temperatures helium may form van der Waals molecules.
Contents
- Known solid phases
- Disodium helide
- Silicates
- Dihelium arsenolite
- Small molecule
- Clathrates
- Fullerites
- Impurity helium condensates
- Impurity solid helium
- Solid solution
- Nanowires
- Two dimensional ionic crystal
- Known van der Waals molecules
- Known ions
- Ionised clusters
- Helium hydride
- Noble gas
- Metals
- Nonmetals
- Excimers
- Endohedral
- Predicted solids
- Predicted van der Waals molecules
- Predicted ions
- Discredited or unlikely observations
- References
Repulsive forces between helium and other atoms may be overcome by high pressures. Helium has been shown to form a crystalline compound with sodium under pressure. Suitable pressures to force helium into solid combinations could be found inside planets. Clathrates are also possible with helium under pressure in ice, and other small molecules such as nitrogen.
Other ways to make helium reactive are: to convert it into an ion, or to excite an electron to a higher level, allowing it to form excimers. Ionised helium (He+), also known as He II, is a very high energy material able to extract an electron from any other atom. He+ has an electron configuration like hydrogen, so as well as being ionic it can form covalent bonds. Excimers do not last for long, as the molecule containing the higher energy level helium atom can rapidly decay back to a repulsive ground state, where the two atoms making up the bond repel. However, in some locations such as helium white dwarfs, conditions may be suitable to rapidly form excited helium atoms. The excited helium atom has a 1s electron promoted to 2s. This requires 460 kcal/gram of energy, which can be supplied by electron impact, or electric discharge. The 2s excited electron state resembles that of the lithium atom.
Known solid phases
Most solid combinations of helium with other substances require high pressure. Helium does not bond with the other atoms, but the substances can have a well defined crystal structure.
Disodium helide
Na2He is a compound of helium and sodium that is stable at high pressures above 113 gigapascals (1,130,000 bar). Na2He was first predicted using USPEX code. It was predicted to be thermodynamically stable over 160 GPa and dynamically stable over 100 GPa. This means it should be possible to form at the higher pressure and then decompress to 100 GPa, but below that it would decompose. Compared with other binary compounds of other elements and helium, it was predicted to be stable at the lowest pressure of any such combination. So that for example a helium-potassium compound is predicted to require much higher pressures of the order of terapascals. Na2He has a cubic crystal structure, resembling fluorite. At 300 GPa the edge of a unit cell of the crystal has a=3.95 Å. Each unit cell contains four helium atoms on the centre of the cube faces and corners, and eight sodium atoms at coordinates a quarter cell in from each face. Double electrons (2e−) are positioned on each edge and the centre of the unit cell. Each pair of electrons is spin paired. The presence of these isolated electrons makes this an electride. The helium atoms do not participate in any bonding. However the electron pairs can be considered as an eight-centre two-electron bond. The material was made in a diamond anvil cell at 130 GPa heated to 1,500 K with a laser. Na2He is predicted to be an insulator and transparent. The sodium atoms have the Bader charge of +0.6, the helium charge is -0.15 and the two-electron spots are -1.1. So this phase could be called disodium helium electride. The solid is an electrical insulator and is predicted to be transparent. Na2He melts at a high temperature near 1,500 K, much higher than the melting point of sodium. When decompressed, it can keep its form as low as 113 GPa.
Silicates
Cristobalite He II (SiO2He) is stable between 1.7 and 6.4 GPa. It has a rhombohedral space group R-3c with unit cell dimensions a=9.080 α=31.809° V=184.77 Å3 at 4GPa.
Cristobalite He I (SiO2He) is formed under higher helium pressures over 6.4 GPa. It has a monoclinic space group P21/C with unit cell dimensions a=8.062 b=4.797 c=9.491 Å β=120.43° V=316.47 Å3 at 10 GPa.
Helium penetrates into fused silica at high pressure, reducing its compressibility.
Dihelium arsenolite
Dihelium arsenolite As4O6•2He is stable from pressures over 5 GPa and up to at least 30 GPa. Arsenolite is one of the softest and most compressible minerals.
Small molecule
He(N2)11 is a van der Waals compound with hexagonal crystals. At 10 GPa the unit cell of 22 nitrogen atoms has a unit cell volume of 558 Å3, and about 512 Å3 at 15 GPa. These sizes are around 10 Å3 smaller than the equivalent amount of solid δ-N2 nitrogen at these pressures. The substance is made by compressing nitrogen and helium in a diamond anvil cell.
NeHe2 has a crystal structure of hexagonal MgZn2 type at 13.7 GPa. The unit cell has dimensions a=4.066 Å c=6.616 Å; and at 21.8 GPa a=3.885 Å c=6.328 Å. There are four atoms in each unit cell. It melts at 12.8 GPa and 296 K, stable to over 90 GPa.
Clathrates
Helium clathrates only form under pressure. With ice II at pressures between 280 and 480 MPa a solid helium hydrate with He:H2O ratio of 1:6 exists. Other helium hydrates with the ice-Ih, ice-Ic 1:1, and ice-Ic 2:1 He to H2O ratio have been predicted. Helium clathrate hydrates should be similar to hydrogen clathrate due to the similar size of the hydrogen molecule.
Fullerites
Helium can form intercalation compounds with the fullerites, including buckminsterfullerene C60 and C70. In solid C60 there are spaces between the C60 balls, either tetrahedral or octahedral in shape. Helium can diffuse into the solid fullerite even at one atmosphere pressure. Helium enters the lattice in two stages. The first rapid stage takes a couple of days, and expands the lattice by 0.16% (that is 0.022 Å) filling the larger octahedral sites. The second stage takes thousands of hours to absorb more helium and expands the lattice twice as much again (0.32%) filling the tetrahedaral sites. However the solid C60•3He is not stable and loses helium on a timescale of 340 hours when not under a helium atmosphere. When the helium intercalated fullerite is cooled, it has an orientational phase transition that is 10K higher than for pure solid C60. The actual discontinuous change in volume at that point is smaller, but there are more rapid changes near the transition temperature, perhaps due to varying occupancy of the voids by helium.
Impurity helium condensates
Impurity helium condensates (IHCs) (or impurity helium gels) are deposited as a snow like gel in liquid helium when various atoms or molecules are absorbed on the surface of superfluid helium. Atoms can include H, N, Na, Ne, Ar, Kr, Xe, alkalis or alkaline earths. The impurities form nanoparticle clusters coated with localised helium held by van der Waals force. Helium atoms are unable to move towards or away from the impurity, but perhaps can move perpendicularly around the impurity. The snow like solid is structured like an aerogel. When free atoms are included in the condensate a high energy density can be achieved, up to 860 Jcm−1 or 5 kJg−1. These condensates were first investigated as a possible rocket fuel. The mixtures are given a notation involving square brackets so that [N]/[He] represents a nitrogen atom impurity in helium.
[N]/[He] atomic nitrogen impurity helium is produced when a radio frequency discharge in a nitrogen helium mixture is absorbed into superfluid helium, it can have up to 4% nitrogen atoms included. The substance resembles crumbly snow and condenses and settles from the liquid helium. It also contains variable proportions of N2 molecules. This substance is a high energy solid, with as much power as convention explosives. When it is heated above 2.19K (the lambda point of helium) the solid decomposes and explodes. This substance is not a true compound, but more like a solid solution. E. B. Gordon et al. suggested that this material may exist in 1974. The localised helium shells around an individual atom are termed van der Waals spheres. However the idea that the nitrogen atoms are dispersed in the helium has been replaced by concept of nitrogen atoms attached to the surface of clusters of nitrogen molecules. The energy density of the solid can be increased by pressing it.
Other inert gas impurity helium condensates can also be made from a gas beam into superfluid He. [Ne]/[He] decomposes at 8.5 K with release of heat and formation of solid neon. Its composition approximates NeHe16.
[Ar]/[He] contains 40-60 He per Ar
[Kr]/[He] contains 40-60 He per Kr and is stable up to 20 K.
[Xe]/[He] contains 40-60 He per Xe
[N2]/[He] contains 12-17 He atoms per N2 molecule. It is stable up to 13 K
[N]/[Ne]/[He] Formed from gas beam generated from a radio-frequency electric discharge in mixtures of neon, nitrogen and helium into superfluid He. Additional inert gas stabilises more nitrogen atoms. It decomposes around 7 K with a blue green light flash. Excited nitrogen atoms in the N(2D) state can be relative long lasting, up to hours, and give off a green luminescence.
[H2]/[He], or [D2]/[He] when dihydrogen or dideuterium is absorbed into superfluid helium, filaments are formed. When enough of these form the solid resembles cotton, rather than snow. Using H2 results in the product floating and stopping further production, but with deuterium, or a half-half mixture, it can sink and accumulate. Atomic hydrogen in impurity helium decays fairly rapidly due to quantum tunneling (H + H → H2). Atomic deuterium dimerises slower (D + D → D2), but reacts very quickly with any diprotium present. (D + H2 → HD + H). Atomic hydrogen solids are further stabilised by other noble gases such as krypton. Lowering temperatures into the millikelvin range can prolong the lifetime of atomic hydrogen condensates. Condensates containing heavy water or deuterium are under investigation for the production of ultracold neutrons. Other impurity gels have been investigated for producing ultracold neutrons include CD4 (deuterated methane) and C2D5OD. (deuterated ethanol)
The water-helium condensate [H2O]/[He] contains water clusters of several nanometers in diameter, and pores from 8 to 800 nm.
Oxygen O2 impurity helium contains solid oxygen clusters from 1 to 100 nm.
Impurity solid helium
Introducing impurities into solid helium yields a blue solid that melts at a higher temperature than pure He. For cesium the absorption has a peak at 750 nm, and for rubidium, maximal absorption is at 640 nm. These are due to metal clusters with diameters of 10 nm or so. However the low concentration of clusters in this substance should not be sufficient to solidify helium as the amount of metal in the solid is less than billionth that of the impurity helium condensate solids, and liquid helium does not "wet" cesium metal. The solid is possibly due to helium snowballs attached to Cs+ (or Rb+) ions. Free electrons in liquid helium are enclosed in a bubble 17 Å in diameter. Under 25 atmosphere pressure an electron bubble reduces to 11 Å.
Solid solution
Helium can dissolve to a limited extent in hot metal, with concentration proportional to pressure. At atmospheric pressure, 500 °C bismuth can absorb 1 part in a billion; at 649 °C lithium can take 5 parts per billion; and at 482 °C potassium can take 2.9 parts per million (all atom fractions). In nickel there can be 1 in 1010 atoms, and in gold 1 in 107. The supposition is that the higher the melting point the less helium can be dissolved. However, when a liquid metal is quenched, higher concentrations of helium can be left dissolved. So cooled liquid steel can have one part per million of helium. In order to get a helium atom into a metal lattice, a hole has to be formed. The energy to make that hole in the metal is basically the heat of solution.
Nanowires
Gold, copper, rubidium, caesium, or barium atoms evaporated into liquid helium form spider web like structures. Rhenium produces nano flakes. Molybdenum, tungsten, and niobium produce thin nanowires with diameters of 20, 25 and 40 Å. Indium, tin, lead and nickel produce nanowires about 80 Å in diameter. These same four metals also produce smooth spheres about 2 μm across that explode when examined with an electron microscope. Copper, permalloy, and bismuth also make nanowires.
Two-dimensional ionic crystal
Helium II ions (He+) in liquid helium when attracted by an electric field can form a two-dimensional crystal at temperatures below 100 mK. There are about half a trillion ions per square meter just below the surface of the helium. Free electrons float above the helium surface.
Known van der Waals molecules
Known ions
Helium has the highest ionisation energy, so a He+ ion will strip electrons off any other neutral atom or molecule. However it can also then bind to the ion produced. The He+ ion can be studied in gas, or in liquid helium. Its chemistry is not completely boring. For example, He+ can react with SF6 to yield SF+
6 or SF+
5 and atomic fluorine.
Ionised clusters
He+
2 was predicted to exist by Linus Pauling in 1933. It was discovered when doing mass spectroscopy on ionised helium. The dihelium cation is formed by an ionised helium atom combining with a helium atom: He+ + He → He+
2.
The diionised dihelium He2+
2 (1Σ+
g) is in a singlet state. It breaks up He2+
2 → He+ + He+ releasing 200 kcal/mol of energy. It has a barrier to decomposition of 35 kcal/mol and a bond length of 0.70 Å.
The trihelium cation He+
3 is in equilibrium with He+
2 between 135 and 200K
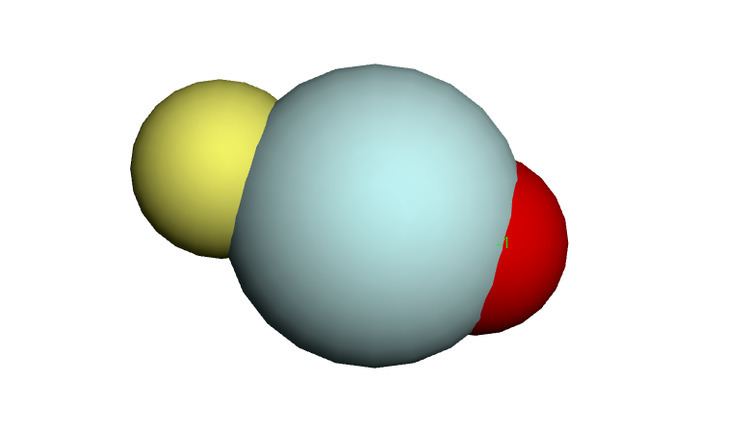
Helium hydride
The helium hydride ion HeH+ has been known since 1925. The protonated dihelium ion He2H+ can be formed when the dihelium cation reacts with dihydrogen: He+
2 + H2 → He2H+ + H. This is believed to be a linear molecule. Larger protonated helium cluster ions exist HenH+ with n from 3 to 14. He6H+ and He13H+ appear to be more common. These can be made by reacting the H+
2 or the H+
3 with gaseous helium.
HeH2+ is unstable in its ground state. But when it is excited to the 2pσ state the molecule is bound with an energy of 20 lcalmol−1. This doubly charged ion has been made by accelerating the helium hydride ion to 900 keV, and firing it in to Argon. It only has a short life of 4 ns.
H2He+ has been made and could occur in nature via H2 + He+ → H2He+.
H3He+
n exists for N from 1 to over 30, and there are also clusters with more hydrogen atoms and helium.
Noble gas
Noble gas cluster ions exist for different noble gases. Singly charged cluster ions containing xenon exist with the formula HenXe+
m, where n and m ≥ 1.
Many different HenKr+ exist with n=1 to 17 at least. HenKr+
2 and HenKr+
3 also exist for many values of n. He12Kr+
2 and He12Kr+
3 ions are commons. These singly charged cluster ions can be made from krypton in helium nanodroplets subject to vacuum ultraviolet radiation.
The Ar+ Argon ion can form many different sized clusters with helium ranging from HeAr+ to He50Ar+, but the most common clusters are He12Ar+ and smaller. These clusters are made by capturing an argon atom in a liquid helium nanodroplet, and then ionising with high speed electrons. He+ is formed, which can transfer charge to argon and then form a cluster ion when the rest of the droplet evaporates.
NeHe+
n can be made by ultraviolet photoionisation. Clusters only contain one neon atom. The number of helium atoms n can vary from 1 to 23, but NeHe+
4 and NeHe+
8 are more likely to be observed.
Doubly charged ions of helium with noble gas atoms also exist including ArHe2+, KrHe2+, and XeHe2+.
Metals
Various metal-helium ions are known.
Alkali metal helide ions are known for all the alkalis. The molecule ground state for the diatomic ions is in the X1Σ+ state. The bond length gets bigger as the periodic table is descended with lengths of 1.96, 2.41, 2.90, 3.10, and 3.38 Å for Li+He, Na+He, K+He, Rb+He, and Cs+He. The dissociation energies are 1.9, 0.9, 0.5, 0.4 and 0.3 kcal/mol, showing bond energy decreases. When the molecule breaks up the positive charge is never on the helium atom.
When there are many helium atoms around, alkali metal ions can attract shells of helium atoms. Clusters can be formed from absorbing metal into helium droplets. The doped droplets are ionised with high speed electrons. For sodium clusters appear with the formula Na+Hen with n from 1 to 26. Na+He is the most common, but Na+He2 is very close in abundance. Na+He8 is much more abundant than clusters with more helium. Na+
2Hen with n from 1 to 20 also appears. Na+
3Hen with small n is also made. For potassium, K+Hen with n up to 28, and K+
2Hen for n from 1 to 20 is formed. K+He and K+He2 are both common, and K+He12 is a bit more commonly formed than other similar sized clusters. Cesium and rubidium cations also form clusters with helium.
Other known metal-helium ions include Cr+He, Co+He, Co+He3, Ni+He, and Ni+He3. PtHe2+; formed by high electric field off platinum surface in helium, VHe2+, HeRh2+ is decomposed in high strength electric field, Ta2+He, Mo2+He, W2+He, Re2+He, Ir2+He, Pt2+He2, W3+He2, W3+He3, and W3+He4.
Nonmetals
HeN+
2 can form at around 4 K from an ion beam of N+
2 into cold helium gas. The energy nneded to break up the molecule is 140 cm−1 which is quite a bit stronger than the van der Waals neutral molecules. HeN+
2 is tough enough to have several vibrational, bending and rotational states. HenN+
2 with n from 2 to 6 have been made by shooting electrons at a supersonically expanding mix of nitrogen and helium.
C60He+ is formed by irradiating C60 with 50eV electrons and then steering ions into cold helium gas. C60He+
2 is also known.
He(OH)+ has been detected, although it is not produced when HTO (tritiated water) decays.
Hen(CO)+ has been detected for values of n from 1 to 12. Also CH3He+, OCHHe+ and NH2He+ have been detected.
Young and Coggiola claimed to make HeC+ by an electric discharge off graphite into helium.
When tritium substituted methane (CH3T) decays, CH3He+ is produced in a very small amount.
The helium formyl cation, HeHCO+ is a linear molecule. It has a vibrational frequency red shifted 12.4 cm−1 compared to HCO+. It can be considered as a deenergized protonation reaction intermediate for the HeH+ + CO → HCO+ + He. HeHCO+ can be produced by a supersonic expansion of a gas mixture of He, CO, and H2, which is hit by a cross beam of electrons. CO and H2 are only supplied at 1% of the helium.
The HeHN+
2 molecule is linear. The He-H bondlength is 1.72 Å. It has an infrared band, due to B-H stretching, with a base at 3158.42 cm−1. The binding energy is 378 cm−1 in the 000 vibrational state, and 431 cm−1 in the 100 vibrational state. He2HN+
2 is also known. One helium atom is linked to a hydrogen, and the other is less tightly bound.
Excimers
The He*
2 excimer is responsible for the Hopfield continuum. Helium also forms an excimer with barium, Ba+He*.
Endohedral
Helium atoms can be trapped inside molecular cages such as the fullerenes He@C60, He@C70, He2@C60 and He2@C70 have all been made using compressed helium and fullerenes.
Other cage like inorganic or organic molecules may also trap helium, for example C8He with He inside a cube., or He@Mo6Cl8F6.
Predicted solids
He(H2O)2 is predicted to form a solid with orthorhomic structure Ibam.
FeHe iron helide was early on claimed to have been found, but the discovery is unlikely. However it is predicted to exist as an interstitial compound under high pressure. It perhaps can exist in dense planetary cores. Freeman Dyson suggested FeHe could exist in neutron star crust material.
Na2HeO is predicted to have a similar structure to Na2He, but with oxygen atoms in the same position as the electron pair, so that it becomes O2−. It would be stable from 13 to 106 GPa. This substance could be a way to store helium in a solid.
La2/3-xLi3xTiO3He is a porous lithium ion conduction perovskite that can contain helium like a clathrate.
Predicted van der Waals molecules
The beryllium oxide helium adduct, HeBeO is believed to be bonded much more strongly than a normal van der Waals molecule with about 5 kcal/mol of binding energy. The bond is enhanced by a dipole induced positive charge on beryllium, and a vacancy in the σ orbital on beryllium where it faces the helium.
Variations on the beryllium oxide adduct include HeBe2O2, RNBeHe including HNBeHe, CH3NBeHe, CH4−xNBeHex, SiH4−xNBeHex, NH3−xNBeHex, PH3−xNBeHex, OH2−xNBeHex, and SH2−xNBeHex.
Hydridohelium fluoride HHeF is predicted to have a lifetime 157 femtoseconds 05 kcal/mol barrier. The lifetime of the deuterium isotopomer is predicted to be much longer due to a greater difficulty of tunneling for deuterium. This molecule's metastability is slated due to electrostatic attraction between HHe+ and F− which increases the barrier to an exothermic breakup. Under pressures over 23 GPa HHeF should be stable.
Calculations for coinage metal fluorides include HeCuF as stable, HeAgF is unstable, HeAuF is predicted, and Ag3He with binding energy 1.4 cm−1, Ag4He binding energy 1.85 cm−1, Au3He binding energy 4.91 cm−1, and Au4He binding energy 5.87 cm−1
HeNaO is predicted.
Calculation for binary van der Waals helium molecules include HeNe, Li4He binding energy 0.008 cm−1, the Li3He is not stable. Na4He binding energy 0.03 cm−1, the Na3He is not stable. Cu3He binding energy 0.90 cm−1, O4He binding energy 5.83 cm−1, S4He binding energy 6.34 cm−1, Se4He binding energy 6.50 cm−1, F4He binding energy 3.85 cm−1, Cl4He binding energy 7.48 cm−1, Br4He binding energy 7.75 cm−1, I4He binding energy 8.40 cm−1, N4He binding energy 2.85 cm−1, P4He binding energy 3.42 cm−1, As4He binding energy 3.49 cm−1, Bi4He binding energy 33.26 cm−1, Si4He binding energy 1.95 cm−1, Ge4He binding energy 2.08 cm−1, CaH4He binding energy 0.96 cm−1, NH4He binding energy 4.42 cm−1, MnH4He binding energy 1.01 cm−1, YbF4He binding energy 5.57 cm−1 I4
2He or I3
2He,
Bonds are predicted to form to nickel with helium as a weak ligand in HeNiCO and HeNiN2.
(HeO)(LiF)2 is predicted to form a planar metastable molecule. 1-Tris(pyrazolyl)borate beryllium and 1-tris(pyrazolyl)borate magnesium are predicted to bind helium at low temperatures. There is also a prediction of a He-O bond in a molecule with caesium fluoride or tetramethyl ammonium fluoride.
LiHe2 is predicted to be in an Efimov state when excited.
Predicted ions
Many ions have been investigated theoretically to see if they could exist. Just about every diatomic cation with helium has been studied. For the diatomic dications, for stability the second ionisation level of the partner atom has to be below the first ionisation level of helium, 24.6 eV. For Li, F, and Ne the ground state is repulsive, so molecules will not form. For N and O the molecule would break up to release He+. However HeBe2+, HeB2+ and HeC2+ are predicted to be stable. Also second row elements from Na to Cl are predicted to have a stable HeX2+ ion.
HeY3+ is predicted to be the lightest stable diatomic triply charged ion. Other possibly thermochemically stable ions include HeZr3+, HeHf3+, HeLa3+, HeNd3+, HeCe3+, HePr3+, HePm3+, HeSm3+, HeGa3+, HeTb3+, HeDy3+, HeHo3+, HeEr3+, HeTm3+, and HeLu3+ where the third ionisation point is below that of helium.
The positronium helide ion PsHe+ should be formed when positrons encounter helium.
The Fluoroheliate FHeO− ion should be stable but salts like LiFHeO are not stable.
Media related to Fluoroheliates at Wikimedia Commons
The lithium hydrohelide cation HLiHe+ is linear in theory. This molecular ion could exist with big bang nucleosynthesis elements. Other hydrohelide cations that exist in theory are HNaHe+ sodium hydrohelide cation, HKHe+ potassium hydrohelide cation, HBeHe2+ beryllium hydrohelide cation, HMgHe2+ magnesium hydrohelide cation, and HCaHe2+ calcium hydrohelide cation.
HeBeO+ is predicted to have a relatively high binding energy of 25 kcal mol−1.
For negative ions the adduct is very weakly bound. Those studied include HeCl−, HeBr−, HeF−, HeO− and HeS−.
2
HHeNH+
3 is predicted to have a C3v symmetry and a H-He bond length of 0.768 Å and He-N 1.830. The energy barrier against decomposition to ammonium is 19.1 kJ/mol with an energy release of 563.4 kJ/mol. Decomposition to hydrohelium ion and ammonium releases 126.2 kJ/mol.
Discredited or unlikely observations
Numerous researchers attempted to create chemical compounds of helium in the early part of the twentieth century. In 1895 L. Troost and L. Ouvrard believed they had witnessed a reaction between magnesium vapour and helium (and also argon) due to the spectrum of helium disappearing from the tube they were passing it through. In 1906, W. Ternant Cooke claimed to have noticed a reaction of helium with cadmium or mercury vapour by observing an increase in the density of the vapour. Zinc vapour did not react with helium.
J. J. Manley claimed to have found gaseous mercury helide HeHg in 1925 HgHe10; publishing the results in Nature, but then had trouble finding a stable composition, and eventually gave up.
Between 1925 and 1940 in Buenos Aires, Horacio Damianovich studied various metal-helium combinations including beryllium (BeHe), iron (FeHe), palladium (PdHe), platinum (Pt3He, bismuth, and uranium. To make these substances, electrical discharges impacted helium into the surface of the metal. Later these were demoted from the status of compounds, to that of alloys.
Platinum helide, Pt3He was discredited by J. G. Waller in 1960.
Palladium helide, PdHe is formed from tritium decay in palladium tritide, the helium (3He) is retained in the solid as a solution.
Boomer claimed the discovery of tungsten helide WHe2 as a black solid. It is formed by way of an electric discharge in helium with a heated tungsten filament. When dissolved in nitric acid or potassium hydroxide, tungstic acid forms and helium escapes in bubbles. The electric discharge had a current of 5 mA and 1000 V at a pressure between 0.05 and 0.5 mm Hg for the helium. Functional electrolysis currents are from 2-20 mA, and 5-10 mA works best. The process works slowly at 200 V. and 0.02 mm Hg of mercury vapour accelerates tungsten evaporation by five times. The search for this was suggested by Ernest Rutherford. It was discredited by J. G. Waller in 1960. Boomer also studied mercury, iodine, sulfur, and phosphorus combinations with helium. Mercury and iodine helium combinations decomposed around -70 °C Sulfur and phosphorus helium combinations decomposed around -120 °C
H. Krefft and R. Rompe claimed reactions between helium and sodium. potassium, zinc, rubidium, indium, and thallium.
