Formula P2I4 Appearance Orange crystalline solid | Molar mass 569.57 g/mol | |
 | ||
Related compounds | ||
Diphosphorus tetraiodide is an orange crystalline solid with the formula P2I4. It has been used as a reducing agent in organic chemistry. It is a rare example of a compound with phosphorus in the +2 oxidation state, and can be classified as a subhalide of phosphorus. It is the most stable of the diphosphorus tetrahalides.
Contents
Synthesis and structure
Diphosphorus tetraiodide is easily generated by the disproportionation of phosphorus triiodide in dry ether:
2 PI3 → P2I4 + I2It can also be obtained by treating phosphorus trichloride and potassium iodide in anhydrous conditions.
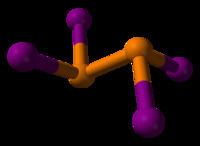
The compound adopts a centrosymmetric structure with a P-P bond of 2.230 Å.
Inorganic chemistry
Diphosphorus tetraiodide reacts with bromine to form mixtures PI3−xBrx. With sulfur, it is oxidized to P2S2I4, retaining the P-P bond.
Organic chemistry
Diphosphorus tetraiodide is used in organic synthesis mainly as a deoxygenating agent. It is used for deprotecting acetals and ketals to aldehydes and ketones, and for converting epoxides into alkenes and aldoximes into nitriles. It can also cyclize 2-aminoalcohols to aziridines and to convert α,β-unsaturated carboxylic acids to α,β-unsaturated bromides.
As foreshadowed by the work of Bertholet in 1855, diphosphorus tetraiodide is used in the Kuhn–Winterstein reaction, the conversion of glycols to alkenes.
