Symbol Peptidase_C1 Pfam clan CL0125 SMART SM00645 | Pfam PF00112 InterPro IPR000668 PROSITE PDOC00126 | |
Serine cysteine proteases
Cysteine proteases, also known as thiol proteases, are enzymes that degrade proteins. These proteases share a common catalytic mechanism that involves a nucleophilic cysteine thiol in a catalytic triad or dyad.
Contents
- Serine cysteine proteases
- Medical vocabulary what does cysteine proteases mean
- Classification
- Catalytic mechanism
- Biological importance
- Regulation
- Potential pharmaceuticals
- Other
- References
Cysteine proteases are commonly encountered in fruits including the papaya, pineapple, fig and kiwifruit. The proportion of protease tends to be higher when the fruit is unripe. In fact, dozens of latices of different plant families are known to contain cysteine proteases. Cysteine proteases are used as an ingredient in meat tenderizers.
Medical vocabulary what does cysteine proteases mean
Classification

The MEROPS protease classification system counts 14 superfamilies plus several currently unassigned families (as of 2013) each containing many families. Each superfamily uses the catalytic triad or dyad in a different protein fold and so represent convergent evolution of the catalytic mechanism.
For superfamilies, P = superfamily containing a mixture of nucleophile class families, C = purely cysteine proteases. superfamily. Within each superfamily, families are designated by their catalytic nucleophile (C = cysteine proteases).
Families of Cysteine proteases
Catalytic mechanism
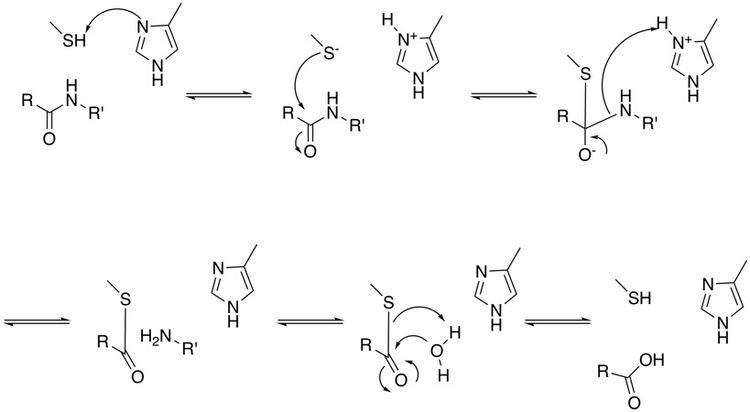
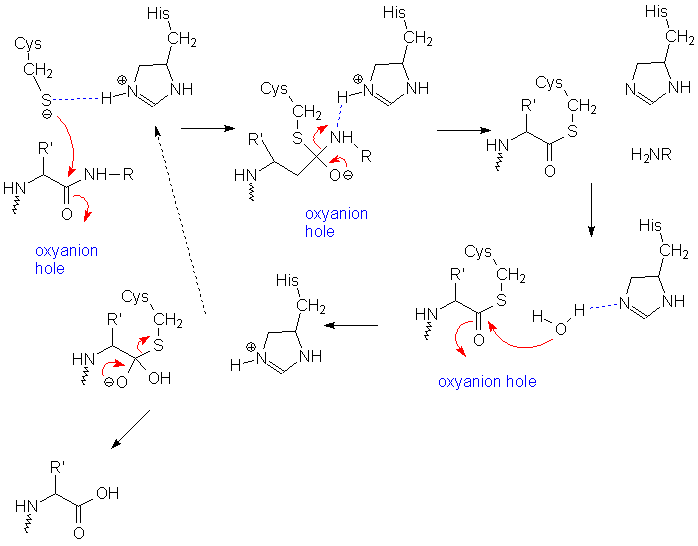
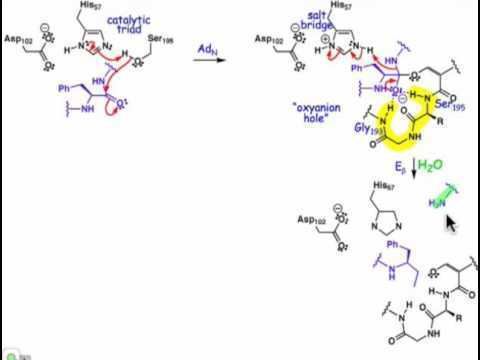
The first step in the reaction mechanism by which cysteine proteases catalyze the hydrolysis of peptide bonds is deprotonation of a thiol in the enzyme's active site by an adjacent amino acid with a basic side chain, usually a histidine residue. The next step is nucleophilic attack by the deprotonated cysteine's anionic sulfur on the substrate carbonyl carbon. In this step, a fragment of the substrate is released with an amine terminus, the histidine residue in the protease is restored to its deprotonated form, and a thioester intermediate linking the new carboxy-terminus of the substrate to the cysteine thiol is formed. Therefore, they are also sometimes referred to as thiol proteases. The thioester bond is subsequently hydrolyzed to generate a carboxylic acid moiety on the remaining substrate fragment, while regenerating the free enzyme.
Biological importance
Cysteine proteases play multi-faceted roles, virtually in every aspect of physiology and development. In plants they are important in growth and development and in accumulation and mobilization of storage proteins such as in seeds. In addition, they are involved in signalling pathways and in the response to biotic and abiotic stresses. In humans and other animals, they are responsible for senescence and apoptosis (programmed cell death), MHC class II immune responses, prohormone processing, and extracellular matrix remodeling important to bone development. The ability of macrophages and other cells to mobilize elastolytic cysteine proteases to their surfaces under specialized conditions may also lead to accelerated collagen and elastin degradation at sites of inflammation in diseases such as atherosclerosis and emphysema. Several viruses (e.g. polio, hepatitis C) express their entire genome as a singe massive polyprotein and use a protease to cleave it into functional units (e.g. Tobacco Etch Virus protease).
Regulation
Proteases are usually synthesized as large precursor proteins called zymogens, such as the serine protease precursors trypsinogen and chymotrypsinogen, and the aspartic protease precursor pepsinogen. The protease is activated by removal of an inhibitory segment or protein. Activation occurs once the protease is delivered to a specific intracellular compartment (e.g. lysosome) or extracellular environment (e.g. stomach). This system prevents the cell that produces the protease from being damaged by it.
Protease inhibitors are usually proteins with domains that enter or block a protease active site to prevent substrate access. In competitive inhibition, the inhibitor binds to the active site, thus preventing enzyme-substrate interaction. In non-competitive inhibition, the inhibitor binds to an allosteric site, which alters the active site and makes it inaccessible to the substrate.
Examples of protease inhibitors include:
Potential pharmaceuticals
Currently there is no widespread use of cysteine proteases as approved and effective anthelmintics but research into the subject is a promising field of study. Plant cysteine proteases isolated from these plants have been found to have high proteolytic activities that are known to digest nematode cuticles, with very low toxicity. Successful results have been reported against nematodes such as Heligmosomoides bakeri, Trichinella spiralis, Nippostrongylus brasiliensis, Trichuris muris, and Ancylostoma ceylanicum; the tapeworm Rodentolepis microstoma, and the porcine acanthocephalan parasite Macracanthorynchus hirundinaceus. A useful property of cysteine proteases is the resistance to acid digestion, allowing possible oral administration. They provide an alternative mechanism of action to current anthelmintics and the development of resistance is thought to be unlikely because it would require a complete change of structure of the helminth cuticle.
In several traditional medicines, the fruits or latex of the papaya, pineapple and fig are widely used for treatment of intestinal worm infections both in humans and livestock.
Other
Cysteine proteases are used as feed additives for livestock to improve the digestibility of protein. They hydrolyse complex proteins into simple amino acids through gut.
