Trade names Epidiolex Biological half-life 9 h | ATC code None CAS ID 13956-29-1 | |
 | ||
AHFS/Drugs.com International Drug Names Legal status AU: S4 (Prescription only)CA: Schedule IIUK: POM (Prescription only) or Dietary SupplementUS: Schedule I Bioavailability 13–19% (oral), 11–45% (mean 31%; inhaled) | ||
Doctors speak out on the benefits of cbd cannabidiol
Cannabidiol (INN) (CBD) is one of at least 113 active cannabinoids identified in cannabis. It is a major phytocannabinoid, accounting for up to 40% of the plant's extract. CBD is considered to have a wide scope of potential medical applications – due to clinical reports showing the lack of side effects, particularly a lack of psychoactivity (as is typically associated with ∆9-THC), and non-interference with several psychomotor learning and psychological functions.
Contents
- Doctors speak out on the benefits of cbd cannabidiol
- Epilepsy
- Psychosis
- Safety
- Pharmacodynamics
- Pharmacokinetic interactions
- Pharmaceutical preparations
- Chemistry
- Biosynthesis
- Isomerism
- Natural sources
- Legal status
- US
- Australia
- Canada
- UK
- Europe
- References
Epilepsy
Dravet syndrome is a rare form of epilepsy that is difficult to treat. It is a catastrophic form of intractable epilepsy that begins in infancy. Initial seizures are most often prolonged events and in the second year of life other seizure types begin to emerge. A number of high profile and anecdotal reports have sparked interest in treatment of Dravet syndrome with cannabidiol.
Some cannabis/hemp extract preparations containing CBD are marketed as dietary supplements and claim efficacy against Dravet syndrome. One such preparation is marketed under the tradename Charlotte's Web Hemp Extract.
GW Pharmaceuticals is seeking FDA approval to market a liquid formulation of pure plant-derived CBD, under the trade name Epidiolex (containing 99% cannabidiol and less than 0.10% Δ9-THC) as a treatment for Dravet syndrome. Epidiolex was granted fast-track status and is in late stage trials following positive early results from the drug.
A 2014 review stated that cannabidiol has been claimed, anecdotally, to be of benefit in helping people with epilepsy. Information in the review stated that there is no established mechanism of action and the lack of high-quality evidence in this area precluded conclusions being drawn.
A 2016 review states that because of the poor quality of available data, "no conclusions can be drawn" about the effectiveness of cannabidiol as an epilepsy treatment.
Psychosis
There is tentative evidence that CBD had an anti-psychotic effect, but research in this area is limited.
Safety
CBD safety in humans has been studied in multiple small studies, suggesting that it is well tolerated at doses of up to 1500 mg/day (p.o.) or 30 mg (i.v.).
Pharmacodynamics
Cannabidiol has very low affinity for the cannabinoid CB1 and CB2 receptors but acts as an indirect antagonist of these receptors. While one would assume that this would cause cannabidiol to reduce the effects of THC, it may potentiate THC's effects by increasing CB1 receptor density or through another CB1 receptor-related mechanism. Cannabidiol may also extend the duration of the effects of THC via inhibition of the cytochrome P450 CYP3A and CYP2C enzymes.
Cannabidiol has been found to act as an antagonist of the GPR55, a G protein-coupled receptor and putative cannabinoid receptor that is expressed in the caudate nucleus and putamen. It has also been shown to act as a 5-HT1A receptor partial agonist, and this action may be involved in the antidepressant, anxiolytic, and neuroprotective effects of cannabidiol. It is an allosteric modulator of the μ and δ-opioid receptors as well. Cannabidiol's pharmacological effects have additionally been attributed to PPARγ agonism and intracellular calcium release.
Research suggests that CBD may exert some of its pharmacological action through its inhibition of fatty acid amide hydrolase (FAAH), which may in turn increase the levels of endocannabinoids, such as anandamide, produced by the body. It has also been speculated that some of the metabolites of CBD have pharmacological effects that contribute to the biological activity of CBD.
Pharmacokinetic interactions
There is preclinical evidence to suggest that cannabidiol may reduce THC clearance, modestly increasing THC's plasma concentrations resulting in a greater amount of THC available to receptors, increasing the effect of THC in a dose-dependent manner. Despite this, the available evidence in humans suggests no significant effect of CBD on THC plasma levels.
Pharmaceutical preparations
Nabiximols (USAN, trade name Sativex) is an aerosolized mist for oral administration containing a near 1:1 ratio of CBD and THC. The drug was approved by Canadian authorities in 2005 to alleviate pain associated with multiple sclerosis. Epidiolex, a drug with cannabidiol as its active pharmaceutical ingredient, received orphan drug status in the United States for treatment of Dravet syndrome in July 2015.
Epidiolex is an oil formulation of CBD extracted from the cannabis plant undergoing clinical trials for refractory epilepsy syndromes.
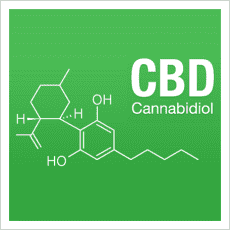
Chemistry
Cannabidiol is insoluble in water but soluble in organic solvents such as pentane. At room temperature, it is a colorless crystalline solid. In strongly basic media and the presence of air, it is oxidized to a quinone. Under acidic conditions it cyclizes to THC. The synthesis of cannabidiol has been accomplished by several research groups.
Biosynthesis
Cannabis produces CBD-carboxylic acid through the same metabolic pathway as THC, until the last step, where CBDA synthase performs catalysis instead of THCA synthase.
Isomerism
Based on: Nagaraja, Kodihalli Nanjappa, Synthesis of delta-3-cannabidiol and the derived rigid analogs, Arizona University 1987.
See also: Tetrahydrocannabinol#Isomerism, Abnormal cannabidiol.
Natural sources
Selective breeding by growers in the USA dramatically lowered the CBD content of cannabis; their customers preferred varietals that were more mind-altering due to a higher THC, lower CBD content. To meet the demands of medical cannabis patients, growers are currently developing more CBD-dominant strains.
Legal status
Cannabidiol is not scheduled by the Convention on Psychotropic Substances. CBD does not cause the "high" associated with the ∆9-THC in marijuana. As the legal landscape and understanding about the differences in medical cannabinoids unfolds, it will be increasingly important to distinguish “medical marijuana” (with noted varying degrees of psychotropic effects and deficits in executive function) – from “medical CBD”.
Various breeds/strains of "medical marijuana" are found to have a significant variety in the ratios of CBD-to-THC and are known to contain other non-psychotropic cannabinoids. However it is only the amount of ∆9-THC that chemically gives a legal determination as to whether the plant material(s) used for the purposes of extracting CBD are considered hemp, or considered marijuana.
Any psychoactive marijuana, regardless of its CBD content, is derived from the flower (or bud) of the genus cannabis. Non-psychoactive hemp (also commonly-termed industrial hemp), regardless of its CBD content, is any part of the genus cannabis plant, whether growing or not, containing a ∆-9 tetrahydrocannabinol concentration of no more than three-tenths of one percent (0.3%) on a dry weight basis. Certain standards are required for the legal growth and production of hemp. The Colorado Industrial Hemp Program registers growers of industrial hemp and samples crops to verify that the THC concentration does not exceed 0.3% on dry weight basis.
U.S.
A CNN program that featured Charlotte's Web in 2013 brought increased demand for CBD-dominant cannabis across the US. Legislation was passed in Kentucky in 2013 to promote Hemp farming in that state as an economic development program to help tobacco farmers who were losing markets for their crops; the state lobbied for and was able to win a provision in the Agricultural Act of 2014 that legalized hemp production in states like Kentucky.
As of 2015, approximately 11 states had legalized industrial hemp production, including: California, Colorado, Indiana, Maine, Montana, North Dakota, Oregon, South Carolina, North Carolina, Vermont, West Virginia, and Tennessee. Many other states have passed legislation authorizing the cultivation of industrial hemp for pilot projects or studies, including: Connecticut, Delaware, Hawaii, Illinois, Kentucky, Nebraska, and Utah. Additionally, the National Association of State Departments of Agriculture and the National Conference of State Legislatures have both adopted resolutions supporting revisions to the federal rules and regulations authorizing commercial production of industrial hemp.
In 2015 the Drug Enforcement Administration (DEA) eased some of the regulatory requirements for those who are conducting FDA-approved clinical trials on cannabidiol (CBD).
On December 14, 2016 the DEA, in the Federal Register Volume 81, Number 240 clarified that all extracts from Cannabis plants, including hemp, are Schedule I Controlled Substances, or a substance with no recognized medical use. People who sell CBD products and who favor their legalization reacted negatively. The DEA notice was a coding change and not a rescheduling of CBD.
Australia
Prescription Medicine (Schedule 4) for therapeutic use containing 2 per cent (2.0%) or less of other cannabinoids commonly found in cannabis (such as ∆9-THC).
Canada
Cannabidiol is a Schedule II drug in Canada. As such, it is only available with a prescription.
UK
Cannabidiol, in an oral-mucosal spray formulation combined with delta-9-tetrahydrocannabinol, is a prescription product available for relief of severe spasticity due to multiple sclerosis (where other anti-spasmodics have not been effective).
As of the 31st December 2016, products containing cannabidiol that are "used for medical purposes" are classed as medicines by the UK regulatory body, the Medicines and Healthcare products Regulatory Agency (MHRA). As such, if a CBD-containing product is "used for medical purposes"—e.g., its advertising claims a medical benefit—it must have a product license before being sold.
Europe
Cannabidiol is listed in the EU Cosmetics Ingredient Database.
Cannabidiol is listed in the EU Novel Food Catalogue. This listing only applies to isolated or synthetic CBD, not to crude hemp extracts or tinctures narturally containing CBD.
The European Industrial Hemp Association has issued a position paper suggesting regulatory framework in EU.
Several industrial hemp varieties can be legally cultivated in western Europe. A variety such as "Fedora 17" has a cannabinoid profile consistently around 1% cannabidiol (CBD) with THC less than 0.1%.
Although the World Health Organization listed Cannabidiolum in a list of International Nonproprietary Names for Pharmaceutical Substances (INN) on 30 June 2016. French and Spanish versions wrongly mention agonist action of CBD on cannabinoid receptors while the English version says CBD is a cannabinoid receptor antagonist.
