 | ||
The Angeli–Rimini reaction is an organic reaction between an aldehyde and the sulfonamide N-hydroxybenzenesulfonamide in presence of base forming an hydroxamic acid.
Contents
The other reaction product is a sulfinic acid. The reaction was discovered by the two Italian chemists Angelo Angeli and E. Rimini, and was published in 1896.
Chemical test
The reaction is used in a chemical test for the detection of aldehydes in combination with ferric chloride. In this test a few drops of aldehyde containing specimen is dissolved in ethanol, the sulfonamide is added together with some sodium hydroxide solution and then the solution is acidified to Congo red. An added drop of ferric chloride will turn the solution an intense red when aldehyde is present. The sulfonamide can be prepared by reaction of hydroxylamine and benzenesulfonyl chloride in ethanol with potassium metal.
Reaction mechanism
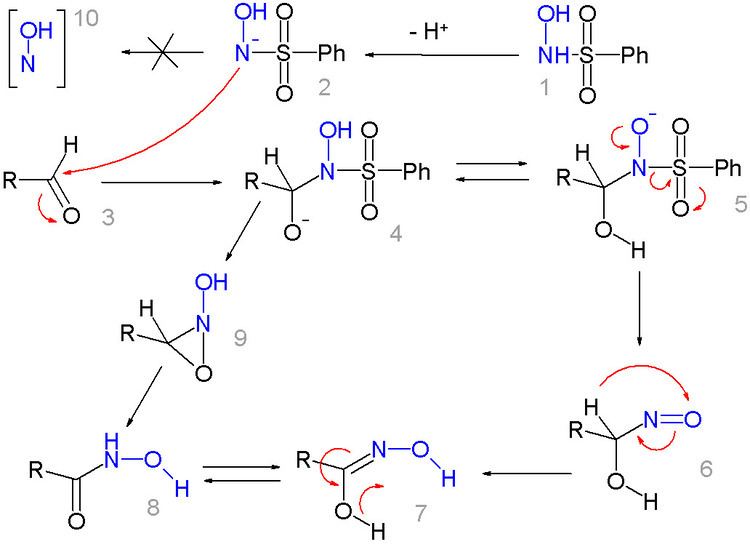
The reaction mechanism for this reaction is not clear and several potential pathways exist. The N-hydroxybenzenesulfonamide 1 or its deprotonated form 2 is a nucleophile in reaction with the aldehyde 3 to intermediate 4. After intramolecular proton exchange to 5 a sulfinic acid anion is split off and hydroxamic acid 8 results through nitrone 6 and intermediate 7. Alternatively aziridine intermediate 9 directly forms the end=product. The formation of the nitrene intermediate 10 is ruled out given the lack of reactivity of the chemical mixture towards simple alkenes.
Scope
The Angeli–Rimini reaction has recently been applied in solid-phase synthesis with the sulfonamide covelently linked to a polystyrene solid support.
