Routes ofadministration Oral CAS Number 749191-14-8 ChemSpider 8188403 | ATC code none PubChem CID 10012829 Molar mass 193.242 g/mol | |
 | ||
Legal status US: Analogue to a Schedule I/II drug (possibly, if human consumption intent can be proven) | ||
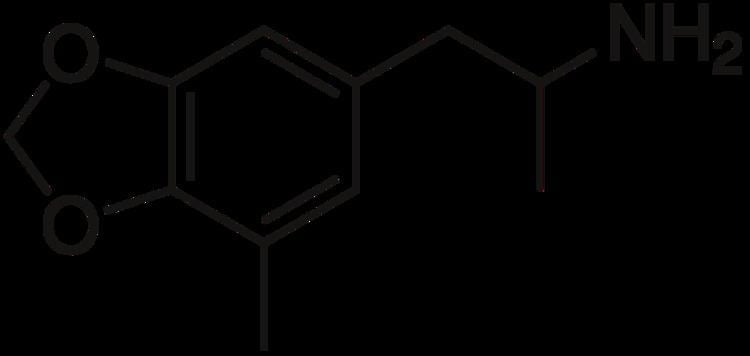
5-Methyl-3,4-methylenedioxyamphetamine (5-Methyl-MDA) is an entactogen and psychedelic designer drug of the amphetamine class. It is a ring-methylated homologue of MDA and a structural isomer of MDMA.
Contents
Effects and research
Drug discrimination studies showed that 5-methyl-MDA substitutes for MDA, MMAI, and LSD, but not amphetamine, suggesting that it produces a mix of entactogen and hallucinogenic effects without any stimulant effects.
5-Methyl-MDA acts as a selective serotonin releasing agent (SSRA) with IC50 values of 107nM, 11,600nM, and 1,494nM for serotonin, dopamine, and norepinephrine efflux. It is over 5x more potent than MDA, with a suitable active dose possibly being around 15–25 mg. Subsequent testing, however, has found that it is not as potent as once thought and is active at at least 100mg. 2-Methyl-MDA is also much more potent than MDA, but is not quite as potent as 5-methyl-MDA. 6-methyl-MDMA (also known as Madam-6) is mostly inactive, likely due to steric hindrance.
Recent research has used data on 2-methyl-MDA and 5-methyl-MDA to help guide computer modeling of the serotonin transporter complex.
Synthesis
The synthesis of 5-methyl-MDA can be found online.
Legal status
5-Methyl-MDA is not scheduled by the United Nations' Convention on Psychotropic Substances.
United States
5-Methyl-MDA is not scheduled at the federal level in the United States, but it is possible that 5-Methyl-MDA could legally be considered an analog of MDA, in which case, sales or possession could potentially be prosecuted under the Federal Analogue Act.
