Formula C8H18 Density 690 kg/m³ | Boiling point 99 °C Appearance Colorless liquid | |
 | ||
Related alkanes | ||
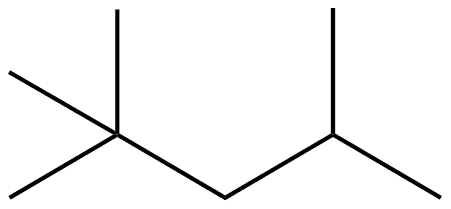
2 2 4 trimethylpentane
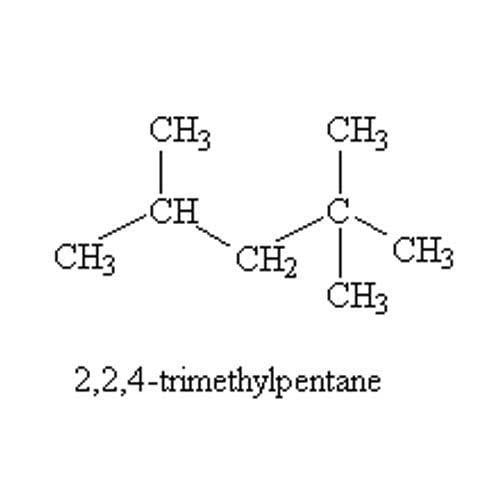
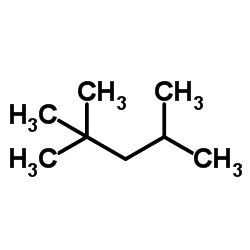
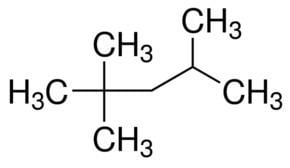
2,2,4-Trimethylpentane, also known as isooctane or iso-octane, is an organic compound with the formula (CH3)3CCH2CH(CH3)2. It is one of several isomers of octane (C8H18). This particular isomer is the standard 100 point on the octane rating scale (the zero point is n-heptane). It is an important component of gasoline, frequently used in relatively large proportions to increase the knock resistance of the fuel.
Contents
Strictly speaking, if the standard meaning of ‘iso’ is followed, the name isooctane should be reserved for the isomer 2-methylheptane. However, 2,2,4-trimethylpentane is by far the most important isomer of octane and so, historically, it has ended up with this name.

Production

Isooctane is produced on a massive scale in the petroleum industry by distillation of petroleum. It can also be produced from isobutylene by dimerization (a variant of alkylation) using an Amberlyst catalyst to produce a mixture of iso-octenes. Hydrogenation of this mixture produces 2,2,4-trimethylpentane.
History
Engine knocking is an unwanted process that can occur during combustion in internal combustion engines. Graham Edgar in 1926 added different amounts of n-heptane and 2,2,4-trimethylpentane to gasoline, and discovered that the knocking stopped when 2,2,4-trimethylpentane was added. This was the origin of the octane rating scale. Test motors using 2,2,4-trimethylpentane gave a certain performance which was standardized as 100 octane. The same test motors, run in the same fashion, using heptane, gave a performance which was standardized as 0 octane. All other compounds and blends of compounds then were graded against these two standards and assigned octane numbers.
Safety
In common with all hydrocarbons, 2,2,4-trimethylpentane is exceedingly flammable in the presence of oxygen, and its vapours readily form explosive mixtures with air.
Also in common with all hydrocarbons, inhalation or ingestion of large quantities of 2,2,4-trimethylpentane is harmful. In rare cases a stronger reaction can occur.