Formula C6H14O2 | ||
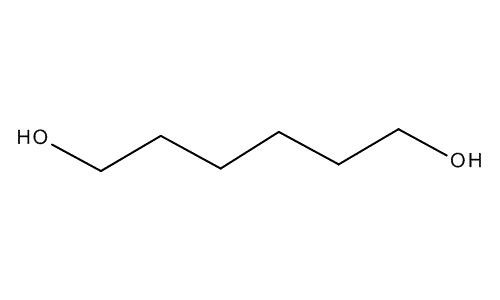 | ||
1,6-Hexanediol (HOCH2(CH2)4CH2OH) is a colorless crystalline solid that melts at 42 °C and boils at 250 °C. It is soluble in water and is hygroscopic.
Contents
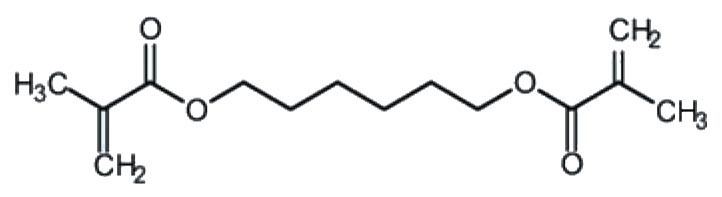
Production
1,6-Hexanediol is prepared industrially by the hydrogenation of adipic acid. Laboratory synthesis can be done by reduction of adipic acid with lithium aluminium hydride, however, since it is cheaply and commercially available, it is usually not synthesized in the laboratory.
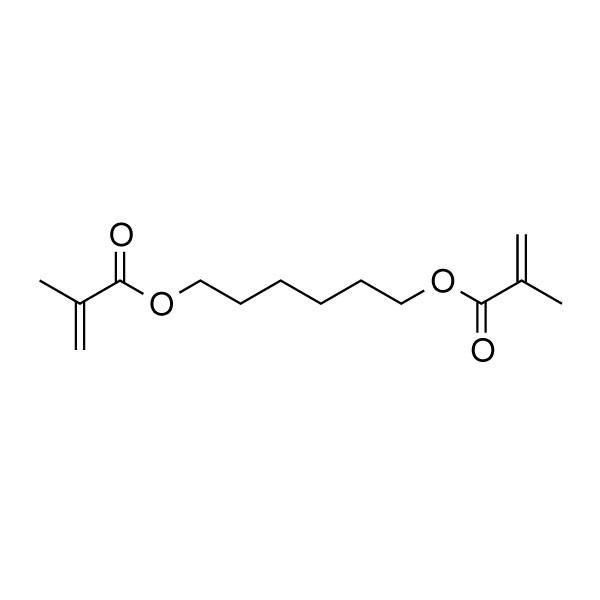
Properties
As 1,6-hexanediol contains the hydroxyl group, it undergoes the typical chemical reactions of alcohols such as dehydration, substitution, esterification.
Dehydration of 1,6-hexanediol gives oxepane, 2-methyltetrahydropyran and 2-ethyltetrahydrofuran. Corresponding thiophene and pyrrolidone can be made by reacting 1,6-hexanediol with hydrogen sulfide and ammonia respectively.
A vast number of monocarboxylic and dicarboxylic esters have been reported, which this reaction is used to synthesize unsaturated polyester resins and polyesters. Polycarbonates can be made from reaction with 1,6-hexanediol with phosgene.
Reaction of the diol with the alkali metals and hydrides give the corresponding alkoxide. Polymerization to polyether can be achieved by using iodine, inorganic acids and organic acids as catalysts.
Uses
1,6-Hexanediol is widely used for industrial polyester and polyurethane production.
1,6-Hexandiol can improve the hardness and flexibility of polyesters as it contains a fairly long hydrocarbon chain. In polyurethanes, it is used as a chain extender, and the resulting modified polyurethane has high resistance to hydrolysis as well as mechanical strength, but with a low glass transition temperature.
It is also an intermediate to acrylics, adhesives, and dyestuffs. Unsaturated polyester resins have also been made from 1,6-hexanediol, along with styrene, maleic anhydride and fumaric acid.
Safety
1,6-Hexanediol has low toxicity and low flammability, and is generally considered as safe. It is not irritating to skin, but may irritate the respiratory tract or mucous membranes. Dust or vapor of the compound can irritate or damage the eyes.
