 | ||
Zmapp and ebola
ZMapp is an experimental biopharmaceutical drug comprising three chimeric monoclonal antibodies under development as a treatment for Ebola virus disease. Two of the three components were originally developed at the Public Health Agency of Canada's National Microbiology Laboratory (NML), and the third at the U.S. Army Medical Research Institute of Infectious Diseases; the cocktail was optimized by Dr. Gary Kobinger, the recently departed branch chief of the NML and is undergoing further development under license by Mapp Biopharmaceutical. Zmapp was first tested in humans during the 2014 West Africa Ebola virus outbreak, but has not been subjected to a randomized controlled trial to determine whether it works, and whether it is safe enough to allow on the market.
Contents
- Zmapp and ebola
- Ebola patients receive zmapp experimental drug
- Medical use
- Mechanism of action
- Chemistry
- History
- MB 003
- ZMAb
- ZMapp
- Clinical trial
- Use during the 201416 Ebola outbreak in West Africa
- Controversy
- References
Ebola patients receive zmapp experimental drug
Medical use
ZMapp is under development as a treatment for Ebola virus disease. It was first used experimentally to treat some people with Ebola virus disease during the 2014 West African Ebola outbreak, but as of August 2014 it had not yet been tested in a clinical trial to support widespread usage in humans; it is not known whether it is effective to treat the disease, nor if it is safe.
Mechanism of action
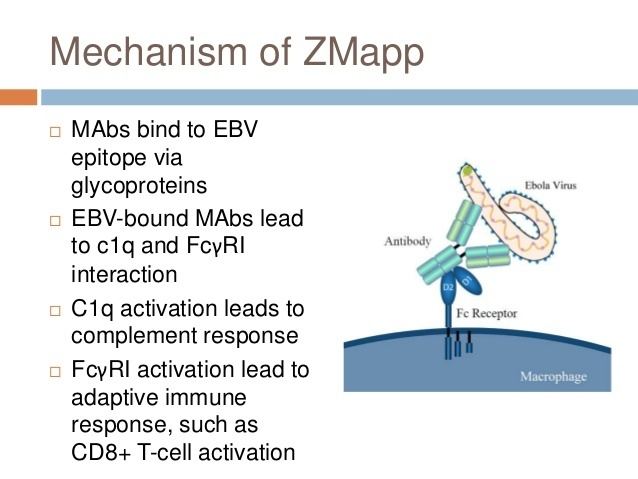
Like intravenous immunoglobulin therapy, ZMapp contains neutralizing antibodies that provide passive immunity to the virus by directly and specifically reacting with it in a "lock and key" fashion.
Chemistry
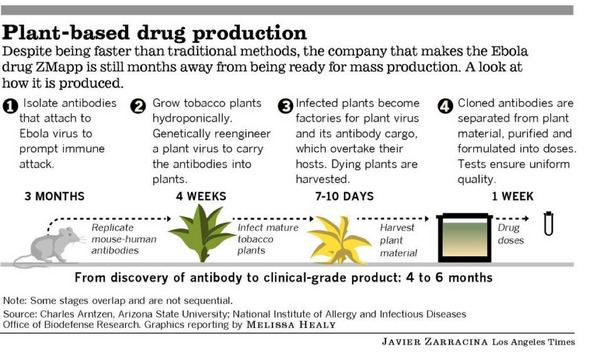
The drug is composed of three monoclonal antibodies (mAbs) that have been chimerized with human constant regions. The components are chimeric monoclonal antibody c13C6 from a previously existing antibody cocktail called "MB-003" and two chimeric mAbs from a different antibody cocktail called ZMab, c2G4 and c4G7. ZMapp is manufactured in the tobacco plant Nicotiana benthamiana in the bioproduction process known as "pharming" by Kentucky BioProcessing, a subsidiary of Reynolds American.
History
Two of the drug's three components were originally developed at the Public Health Agency of Canada's National Microbiology Laboratory (NML), and a third at the U.S. Army Medical Research Institute of Infectious Diseases; the cocktail was optimized by Gary Kobinger, then branch chief of the NML, and is undergoing further development by Leaf Biopharmaceutical (LeafBio, Inc.), a San Diego-based arm of Mapp Biopharmaceutical. LeafBio created ZMapp in collaboration with its parent and Defyrus Inc., each of which had licensed its own cocktail of antibodies, called MB-003 and ZMab.
MB-003

MB-003 is a cocktail of three humanized or human–mouse chimeric mAbs: c13C6, h13F6 and c6D8. A study published in September 2012 found that rhesus macaques infected with Ebola virus (EBOV) survived when receiving MB-003 (mixture of 3 chimeric monoclonal antibodies) one hour after infection. When treated 24 or 48 hours after infection, four of six animals survived and had little to no viremia and few, if any, clinical symptoms.
MB-003 was created by scientists at the U.S. Army Medical Research Institute of Infectious Diseases, Gene Olinger and Jamie Pettitt in collaboration with Mapp Biopharmaceutical with years of funding from US government agencies including the National Institute of Allergy and Infectious Disease, Biomedical Advanced Research and Development Authority, and the Defense Threat Reduction Agency.
ZMAb

ZMAb is a mixture of three mouse mAbs: m1H3, m2G4 and m4G7. A study published in November 2013 found that EBOV-infected macaque monkeys survived after being given a therapy with a combination of three EBOV surface glycoprotein (EBOV-GP)-specific monoclonal antibodies (ZMAb) within 24 hours of infection. The authors concluded that post-exposure treatment resulted in a robust immune response, with good protection for up to 10 weeks and some protection at 13 weeks. ZMab was created by the NML and licensed to Defyrus, a Toronto-based biodefense company, with further funding by the Public Health Agency of Canada.
ZMapp
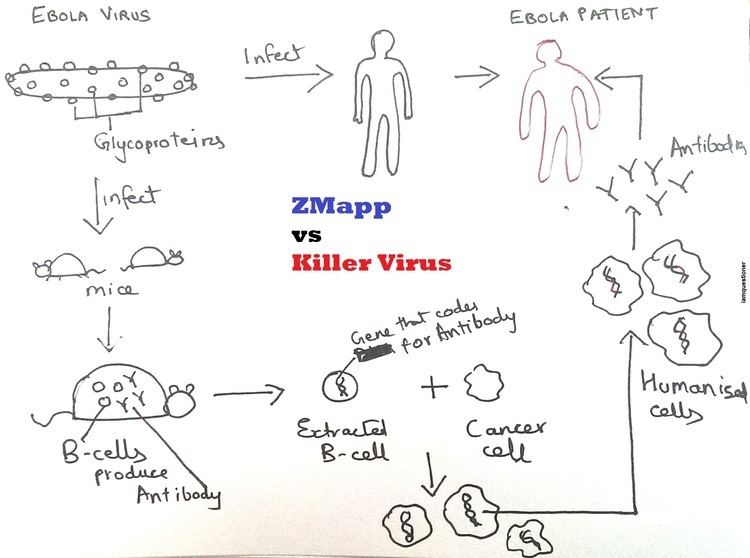
A 2014 paper described how Mapp and its collaborators, including investigators at Public Health Agency of Canada, Kentucky BioProcessing, and the National Institute of Allergy and Infectious Diseases, first chimerized the three antibodies comprising ZMAb, then tested combinations of MB-003 and the chimeric ZMAb antibodies in guinea pigs and then primates to determine the best combination, which turned out to be c13C6 from MB-003 and two chimeric mAbs from ZMAb, c2G4 and c4G7. This is ZMapp.
In an experiment also published in the 2014 paper, 21 rhesus macaque primates were infected with the Kikwit Congolese variant of EBOV. Three primates in the control arm were given a non-functional antibody, and the 18 in the treatment arm were divided into three groups of six. All primates in the treatment arm received three doses of ZMapp, spaced 3 days apart. The first treatment group received its first dose on 3rd day after being infected; the second group on the 4th day after being infected, and the third group, on the 5th day after being infected. All three primates in the control group died; all 18 primates in the treatment arm survived. Mapp then went on to show that ZMapp inhibits replication of a Guinean strain of EBOV in cell cultures.
Mapp remains involved in the production of the drug through its contracts with Kentucky BioProcessing, a subsidiary of Reynolds American. To produce the drug, genes coding for the chimeric mAbs were inserted into viral vectors, and tobacco plants are infected with the viral vector encoding for the antibodies, using Agrobacterium cultures. Subsequently, antibodies are extracted and purified from the plants. Once the genes encoding the chimeric mAbs are in hand, the entire tobacco production cycle is believed to take a few months. The development of these production methods was funded by the U.S. Defense Advanced Research Projects Agency as part of its bio-defense efforts following the 9/11 terrorist attacks.
Clinical trial
The National Institutes of Health announced on 27 February 2015 the commencement of a randomized controlled trial of ZMapp to be conducted in Liberia and the United States.
Use during the 2014–16 Ebola outbreak in West Africa
The FDA allowed two drugs, ZMapp and an RNA interference drug called TKM-Ebola, to be used by Americans who had contracted Ebola virus disease. During 2014, a limited supply of ZMapp was used to treat 7 individuals infected with the Ebola virus; of these 2 died. The outcome is not considered to be statistically significant. Mapp announced in August 2014, that supplies of ZMapp had been exhausted.
Controversy
The lack of drugs and unavailability of experimental treatment in the most affected regions of the West African Ebola virus outbreak spurred some controversy. The fact that the drug was first given to Americans and a European and not to Africans, according to the Los Angeles Times, "provoked outrage, feeding into African perceptions of Western insensitivity and arrogance, with a deep sense of mistrust and betrayal still lingering over the exploitation and abuses of the colonial era". Salim S. Abdool Karim, the director of an AIDS research center in South Africa, placed the issue in the context of the history of exploitation and abuses. Responding to a question on how people might have reacted if ZMapp and other drugs had first been used on Africans, he said "It would have been the front-page screaming headline: 'Africans used as guinea pigs for American drug company's medicine'".
In early August, the World Health Organization called for convening a panel of medical authorities "to consider whether experimental drugs should be more widely released." In a statement, Peter Piot (co-discoverer of the Ebola virus); Jeremy Farrar, the director of the Wellcome Trust; and David Heymann of the Chatham House Center on Global Health Security, called for the release of experimental drugs for affected African nations.
At an August 6, 2014 press conference, Barack Obama, the President of the United States, was questioned regarding whether the cocktail should be fast-tracked for approval or be made available to sick patients outside of the United States. He responded, "I think we've got to let the science guide us. I don't think all the information's in on whether this drug is helpful."
