Appearance White solid Molar mass 207.2836 g/mol Density 4.04 g/cm³ | Formula XeF4 Melting point 117 °C Coordination geometry D4h | |
 | ||
Similar Xenon difluoride, Sulfur tetrafluoride, Xenon hexafluoride | ||
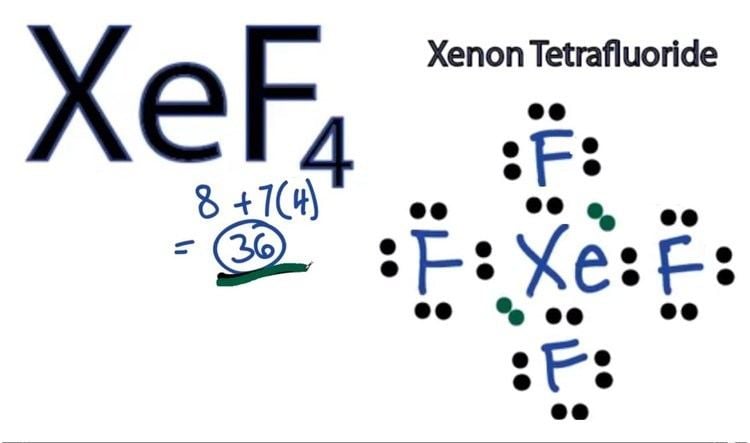
Xenon tetrafluoride xef4 lewis dot structure
Xenon tetrafluoride is a chemical compound with chemical formula XeF
4. It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine, F
2, according to the chemical equation:
Contents
- Xenon tetrafluoride xef4 lewis dot structure
- Lewis dot structure of xef4 xenon tetrafluoride
- Synthesis
- Chemistry
- Applications
- References
2 → XeF
4
This reaction is exothermic, releasing an energy of 251 kJ/mol of xenon.
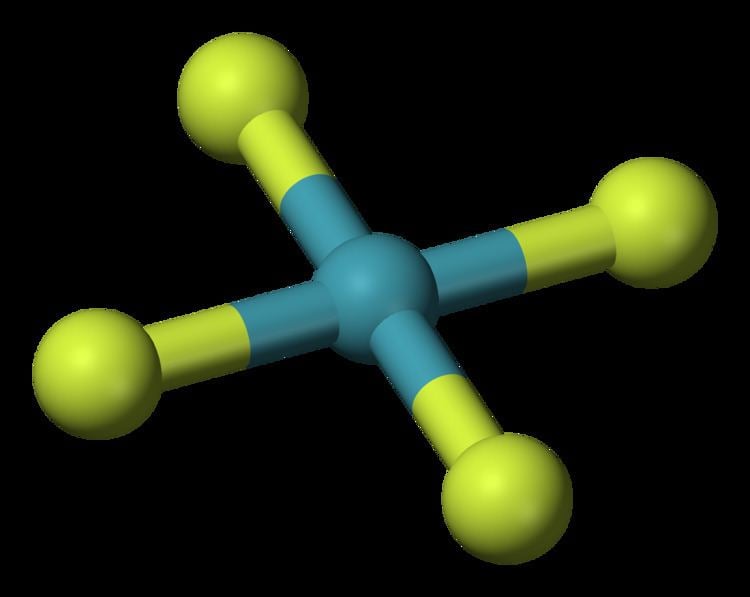
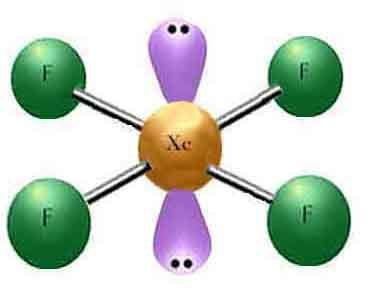
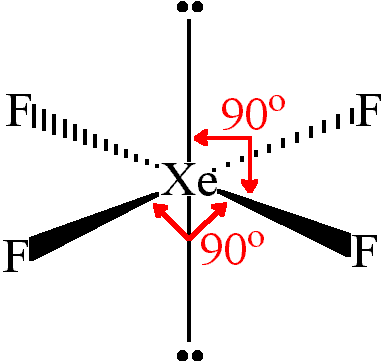
Xenon tetrafluoride is a colorless crystalline substance under ordinary conditions. Its crystalline structure was determined by both NMR spectroscopy and X-ray crystallography in 1963. The structure is square planar, as has been confirmed by neutron diffraction studies, and is justified by VSEPR theory because xenon has two lone pairs of electrons: one above and one below the plane of the molecule.

Xenon tetrafluoride sublimes at a temperature of 115.7 °C (240.26 °F).
The formation of xenon tetrafluoride, like the other xenon fluorides, is exergonic. They are stable at normal temperatures and pressures. All of them readily react with water, releasing pure xenon gas, hydrogen fluoride, and molecular oxygen. This reaction occurs in slightly moist air; hence, all xenon fluorides must be kept in anhydrous atmospheres.
Lewis dot structure of xef4 xenon tetrafluoride
Synthesis
Xenon tetrafluoride is produced by heating a mixture of xenon and fluorine in a 1:5 ratio in a nickel container to 400 °C. Some xenon hexafluoride, XeF
6, is also produced, and this production is increased with an increased fluorine concentration in the input mixture. The nickel is not a catalyst for this reaction; nickel containers are used because they react with fluorine to form a protective, non-peeling layer of nickel fluoride NiF
2 on their interior surfaces.
Chemistry
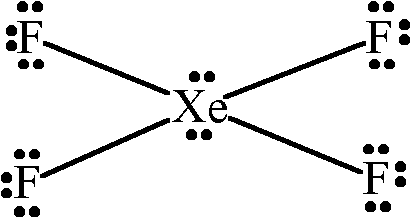
Xenon tetrafluoride is hydrolyzed by water at low temperatures to form elemental xenon, oxygen, hydrofluoric acid, and aqueous xenon trioxide.
Reaction with tetramethylammonium fluoride forms tetramethylammonium pentafluoroxenate, which contains the pentagonal XeF−
5 anion. The XeF−
5 anion is also formed by reaction with caesium fluoride:
4 → CsXeF
5
Reaction with bismuth pentafluoride (BiF
5) forms the XeF+
3 cation:
5 + XeF
4 → XeF3BiF6
The XeF+
3 cation has also been identified in the salt XeF3Sb2F11 by NMR spectroscopy.
At 400 °C, XeF
4 reacts with xenon gas to form XeF
2:
The reaction of xenon tetrafluoride with platinum yields platinum tetrafluoride (PtF
4) and xenon gas:
Applications
Xenon tetrafluoride is used as a decomposition agent of silicone rubber for analysing trace metal impurities in the rubber. XeF
4 reacts with the silicone structure that makes up the backbone of silicone rubber to form simple gaseous products, leaving behind any content of metal impurities.
