 | ||
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae, wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side. The coefficients next to the symbols and formulae of entities are the absolute values of the stoichiometric numbers. The first chemical equation was diagrammed by Jean Beguin in 1615.
Contents
Form
A chemical equation consists of the chemical formulas of the reactants (the starting substances) and the chemical formula of the products (substances formed in the chemical reaction). The two are separated by an arrow symbol (
As an example, the equation for the reaction of hydrochloric acid with sodium can be denoted:
2 HCl + 2 Na → 2 NaCl + H2
This equation would be read as "two HCl plus two Na yields two NaCl and H two." But, for equations involving complex chemicals, rather than reading the letter and its subscript, the chemical formulas are read using IUPAC nomenclature. Using IUPAC nomenclature, this equation would be read as "hydrochloric acid plus sodium yields sodium chloride and hydrogen gas."
This equation indicates that sodium and HCl react to form NaCl and H2. It also indicates that two sodium molecules are required for every two hydrochloric acid molecules and the reaction will form two sodium chloride molecules and one diatomic molecule of hydrogen gas molecule for every two hydrochloric acid and two sodium molecules that react. The stoichiometric coefficients (the numbers in front of the chemical formulas) result from the law of conservation of mass and the law of conservation of charge (see "Balancing Chemical Equation" section below for more information).
Common symbols
Symbols are used to differentiate between different types of reactions. To denote the type of reaction:
The physical state of chemicals is also very commonly stated in parentheses after the chemical symbol, especially for ionic reactions. When stating physical state, (s) denotes a solid, (l) denotes a liquid, (g) denotes a gas and (aq) denotes an aqueous solution.
If the reaction requires energy, it is indicated above the arrow. A capital Greek letter delta (
Balancing chemical equations
The law of conservation of mass dictates that the quantity of each element does not change in a chemical reaction. Thus, each side of the chemical equation must represent the same quantity of any particular element. Likewise, the charge is conserved in a chemical reaction. Therefore, the same charge must be present on both sides of the balanced equation.
One balances a chemical equation by changing the scalar number for each chemical formula. Simple chemical equations can be balanced by inspection, that is, by trial and error. Another technique involves solving a system of linear equations.
Balanced equations are written with smallest whole-number coefficients. If there is no coefficient before a chemical formula, the coefficient 1 is understood.
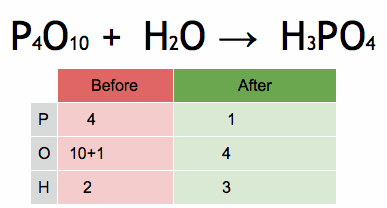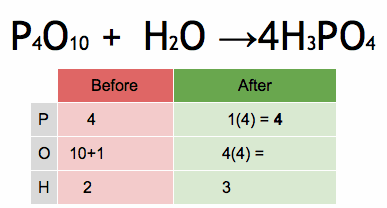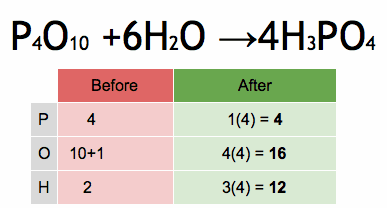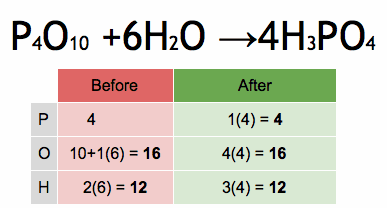
The method of inspection can be outlined as putting a coefficient of 1 in front of the most complex chemical formula and putting the other coefficients before everything else such that both sides of the arrows have the same number of each atom. If any fractional coefficient exists, multiply every coefficient with the smallest number required to make them whole, typically the denominator of the fractional coefficient for a reaction with a single fractional coefficient.
As an example, seen in the above image, the burning of methane would be balanced by putting a coefficient of 1 before the CH4:
1 CH4 + O2 → CO2 + H2OSince there is one carbon on each side of the arrow, the first atom (carbon) is balanced.
Looking at the next atom (hydrogen), the right-hand side has two atoms, while the left-hand side has four. To balance the hydrogens, 2 goes in front of the H2O, which yields:
1 CH4 + O2 → CO2 + 2 H2OInspection of the last atom to be balanced (oxygen) shows that the right-hand side has four atoms, while the left-hand side has two. It can be balanced by putting a 2 before O2, giving the balanced equation:
CH4 + 2 O2 → CO2 + 2 H2OThis equation does not have any coefficients in front of CH4 and CO2, since a coefficient of 1 is dropped.
Ionic equations
An ionic equation is a chemical equation in which electrolytes are written as dissociated ions. Ionic equations are used for single and double displacement reactions that occur in aqueous solutions. For example, in the following precipitation reaction:
the full ionic equation is:
In this reaction, the Ca2+ and the NO3− ions remain in solution and are not part of the reaction. That is, these ions are identical on both the reactant and product side of the chemical equation. Because such ions do not participate in the reaction, they are called spectator ions. A net ionic equation is the full ionic equation from which the spectator ions have been removed. The net ionic equation of the proceeding reactions is:
or, in reduced balanced form,
In a neutralization or acid/base reaction, the net ionic equation will usually be:
H+(aq) + OH−(aq) → H2O(l)There are a few acid/base reactions that produce a precipitate in addition to the water molecule shown above. An example is the reaction of barium hydroxide with phosphoric acid, which produces not only water but also the insoluble salt barium phosphate. In this reaction, there are no spectator ions, so the net ionic equation is the same as the full ionic equation.
Double displacement reactions that feature a carbonate reacting with an acid have the net ionic equation:
If every ion is a "spectator ion" then there was no reaction, and the net ionic equation is null.
