Appearance Colorless liquid Molar mass 86.09 g/mol Density 934 kg/m³ | Formula C4H6O2 Boiling point 72.7 °C | |
 | ||
Ethylene vinyl acetate
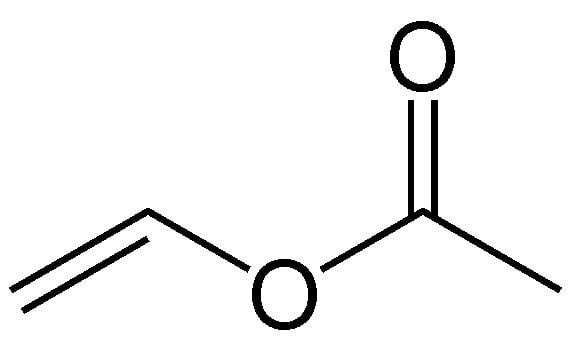
Vinyl acetate is an organic compound with the formula CH3CO2CHCH2. A colorless liquid with a pungent odor, it is the precursor to polyvinyl acetate, an important polymer in industry. Unlike many other acetate esters, the odor of vinyl acetate is thoroughly disagreeable and is not used to any substantial degree as an odorant.
Contents
- Ethylene vinyl acetate
- Production
- Preparation
- Polymerization
- Other reactions
- Toxicity evaluation
- References
Production
The worldwide production capacity of vinyl acetate monomer (VAM) was estimated at 6,154,000 tonnes/annum in 2007, with most capacity concentrated in the United States (1,585,000 all in Texas), China (1,261,000), Japan (725,000) and Taiwan (650,000). The average list price for 2008 was $1600/tonne. Celanese is the largest producer (ca 25% of the worldwide capacity), while other significant producers include China Petrochemical Corporation (7%), Chang Chun Group (6%) and LyondellBasell (5%).
It is a key ingredient in furniture glue.
Preparation
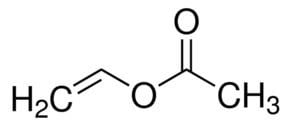
The major industrial route involves the reaction of ethylene and acetic acid with oxygen in the presence of a palladium catalyst.
C2H4 + CH3CO2H + 1⁄2 O2 → CH3CO2CHCH2 + H2OThe main side reaction is the combustion of organic precursors. Vinyl acetate was once prepared by hydroesterification. This method involves the gas-phase addition of acetic acid to acetylene in the presence of metal catalysts. By this route, using mercury(II) catalysts, vinyl acetate was first prepared by Klatte in 1912. Another route to vinyl acetate involves thermal decomposition of ethylidene diacetate:
(CH3CO2)2CHCH3 → CH3CO2CHCH2 + CH3CO2HPolymerization
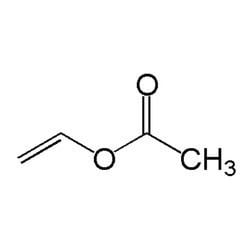
It can be polymerized to give polyvinyl acetate (PVA). With other monomers can be used to prepare copolymers such as ethylene-vinyl acetate (EVA), vinyl acetate-acrylic acid (VA/AA), polyvinyl chloride acetate (PVCA), and polyvinylpyrrolidone (Vp/Va Copolymer, used in hair gels). Due to the instability of the radical, attempts to control the polymerization via most 'living/controlled' radical processes have proved problematic. However, RAFT (or more specifically MADIX) polymerization offers a convenient method of controlling the synthesis of PVA by the addition of a xanthate or a dithiocarbamate chain transfer agent.
Other reactions
Vinyl acetate undergoes many of the reactions anticipated for an alkene and an ester. Bromine adds to give the dibromide. Hydrogen halides add to give 1-haloethyl acetates, which cannot be generated by other methods because of the non-availability of the corresponding halo-alcohols. Acetic acid adds in the presence of palladium catalysts to give ethylidene diacetate, CH3CH(OAc)2. It undergoes transesterification with a variety of carboxylic acids. The alkene also undergoes Diels-Alder and 2+2 cycloadditions.
Toxicity evaluation
On January 31, 2009, the Government of Canada's final assessment concluded that exposure to vinyl acetate is not considered to be harmful to human health. This decision under the Canadian Environmental Protection Act (CEPA) was based on new information received during the public comment period, as well as more recent information from the risk assessment conducted by the European Union.
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.
