Related compounds Molar mass 121.847 g/mol Density 3.23 g/cm³ Pubchem 66355 | Formula VCl2 Boiling point 1,377 °C Appearance pale green solid | |
 | ||
Vanadium(II) chloride is the inorganic compound with the formula VCl2, and is the most reduced vanadium chloride. Vanadium(II) chloride is an apple-green solid that dissolves in water to give purple solutions.
Contents
Preparation, properties, and related compounds
Solid VCl2 is prepared by thermal decomposition of VCl3, which leaves a residue of VCl2:
2 VCl3 → VCl2 + VCl4VCl2 dissolves in water to give the purple hexaaquo ion [V(H2O)6]2+. Evaporation of such solutions produces crystals of [V(H2O)6]Cl2.
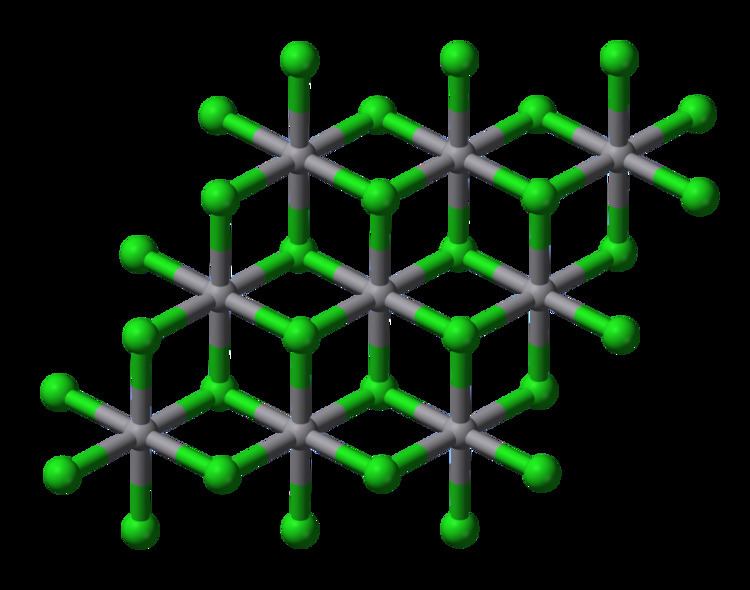
Structure
Solid VCl2 adopts the cadmium iodide structure, featuring octahedral coordination geometry. VBr2 and VI2 are structurally and chemically similar to the dichloride. All have the d3 configuration, with a quartet ground state, akin to Cr(III).
Vanadium dichloride is a powerful reducing species, being able to convert sulfoxides to sulfides, organic azides to amines, as well as reductively coupling some alkyl halides.
