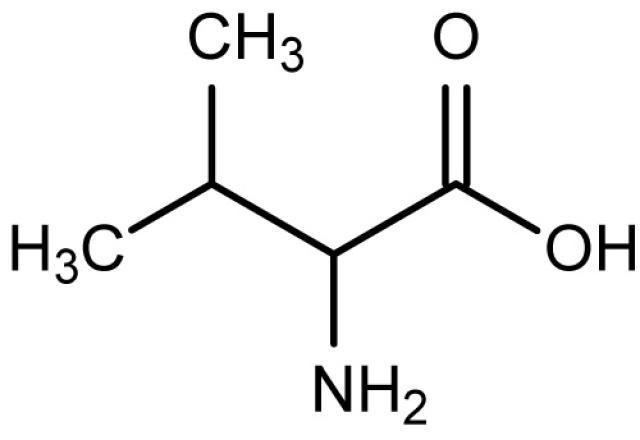
Formula C5H11NO2 Melting point 298 °C Density 1.32 g/cm³ | Molar mass 117.151 g/mol IUPAC ID Valine Soluble in Water | |
 | ||
Thermodynamicdata Phase behavioursolid–liquid–gas | ||
Valine synthesis synthesis of amino acids
Valine (abbreviated as Val or V) encoded by the codons GUU, GUC, GUA, and GUG is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isopropyl variable group, classifying it as a non-polar amino acid. It is essential in humans, meaning the body cannot synthesize it and thus it must be obtained from the diet. Human dietary sources are any proteinaceous foods such as meats, dairy products, soy products, beans and legumes.
Contents
- Valine synthesis synthesis of amino acids
- Valine meaning
- History and etymology
- Nomenclature
- Biosynthesis
- Metabolism of L Valine
- Synthesis
- Valine and insulin resistance
- Valine and hematopoetic stem cells
- References
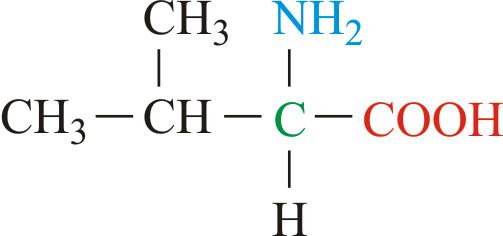
Along with leucine and isoleucine, valine is a branched-chain amino acid. In sickle-cell disease, valine substitutes for the hydrophilic amino acid glutamic acid in β-globin. Because valine is hydrophobic, the hemoglobin is prone to abnormal aggregation.
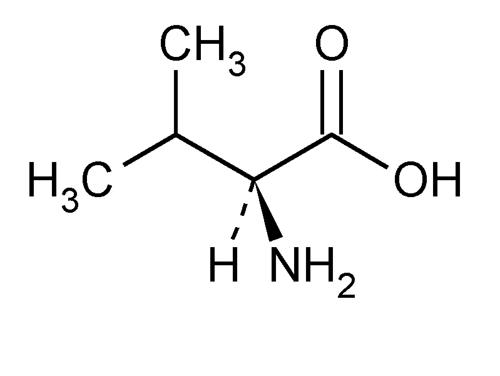
Valine meaning
History and etymology
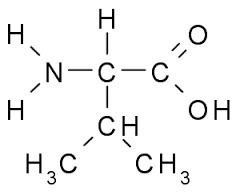
Valine was first isolated from casein in 1901 by Hermann Emil Fischer. The name valine comes from valeric acid, which in turn is named after the plant valerian due to the presence of the acid in the roots of the plant.
Nomenclature
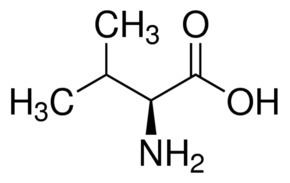
According to IUPAC, carbon atoms forming valine are numbered sequentially starting from 1 denoting the carboxyl carbon, whereas 4 and 4' denote the two terminal methyl carbons.
Biosynthesis
Valine is an essential amino acid, hence it must be ingested, usually as a component of proteins. It is synthesized in plants via several steps starting from pyruvic acid. The initial part of the pathway also leads to leucine. The intermediate α-ketoisovalerate undergoes reductive amination with glutamate. Enzymes involved in this biosynthesis include:
- Acetolactate synthase (also known as acetohydroxy acid synthase)
- Acetohydroxy acid isomeroreductase
- Dihydroxyacid dehydratase
- Valine aminotransferase
Metabolism of L-Valine
L-Valine --> alpha-Ketoisovaleric acid --> Isobutyryl-CoA --> Methylmalonate semialdehyde --> Methylmalonyl-CoA (a form) -->
Methylmolonyl-CoA (b form) --> Succinyl-CoA , which then can enter the Citric Acid Cycle.
Transamination of Valine is by Glutamate-alpha-ketoisovalerate transaminase, oxidative decarboxylation of the product is like that done to Pyruvate, Vitamin B-12 is a cofactor in the isomerization of Methylmalonyl-CoA to Succinyl-CoA
Source: Biochemistry, 8th edition, by James M. Orten and Otto W. Neuhaus, (1970) pages 367-368.
Synthesis
Racemic valine can be synthesized by bromination of isovaleric acid followed by amination of the α-bromo derivative
HO2CCH2CH(CH3)2 + Br2 → HO2CCHBrCH(CH3)2 + HBrHO2CCHBrCH(CH3)2 + 2 NH3 → HO2CCH(NH2)CH(CH3)2 + NH4BrValine and insulin resistance
Valine, as well as other branched-chain amino acids, are associated with insulin resistance, as higher levels of valine are observed in the blood of diabetic mice, rats, and humans. Mice fed a valine free diet for one day have improved insulin sensitivity, and feeding of a valine free diet for one week significantly decreases blood glucose levels. The valine catabolite 3-hydroxyisobutyrate promotes skeletal muscle insulin resistance in mice by stimulating fatty acid uptake into muscle and lipid accumulation. In humans, a protein restricted diet lowers blood levels of valine and decreases fasting blood glucose levels.
Valine and hematopoetic stem cells
Experiments in mice have shown that dietary valine is essential for hematopoetic stem cell self-renewal. Dietary valine restriction selectively depletes long-term repopulating Hematopoetic Stem Cells (HSC) in mouse Bone Marrow (BM). Successful stem cell transplantation was achieved in mice without irradiation after 3 weeks on a -Val diet. Long term survival of the transplanted mice was achieved when valine was returned to the diet gradually over a 2 week period to avoid refeeding syndrome.
