Molar mass 450.745 g/mol | Density 3.6 g/cm³ | |
 | ||
Appearance dark green crystalline solid | ||
Uranium hexachloride is an inorganic chemical compound of uranium in the +6 oxidation state. The chemical compound Uranium hexachloride (UCl6) is a metal halide composed of Uranium and Chlorine. It is a multi-luminescent dark green crystalline solid with a vapor pressure between 1-3 mmHg at 373.15K UCl6 is stable in a vacuum, dry air, nitrogen and helium at room temperature. It is soluble in carbon tetrachloride (CCl4). Compared to the other uranium halides, little is known about UCl6.
Contents
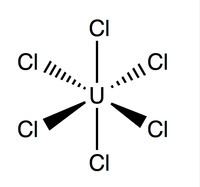
Structure and Bonding
Uranium hexachloride has an octahedral geometry with a point group of Oh. Its lattice (dimensions: 10.95 ± 0.02Å x 6.03 ± 0.01Å) is hexagon in shape with three molecules per cell; the average theoretical U-Cl bond is 2.472Å long (X-ray diffraction experimental U-Cl length is 2.42Å) and the distance between two adjacent chlorine atoms is 3.65Å .
Chemical Properties
Uranium hexachloride is a highly hygroscopic compound and decomposes readily when exposed to ordinary atmospheric conditions. therefore it should be handled in either a vacuum apparatus or in a dry box.
Thermal decomposition
UCl6 is stable up to temperatures between 120oC and 150oC. The decomposition of UCl6 results in a solid phase transition from one crystal form of UCl6 to another more stable form. However the decomposition of gaseous UCl6 produces (UCl5). The Activation energy for this reaction is about 40kcal per mole.
2UCl6 (g) → 2UCl5 (s) + Cl2 (g)
Solubility
UCl6 is not a very soluble compound. It dissolves in CCl4 to give a brown solution. It is slightly soluble in isobutyl bromide and in fluorocarbon (C7F16).
Reaction with Hydrogen Fluoride
When UCl6 is reacted with purified anhydrous liquid Hydrogen Fluoride (HF) at room temperature produces UF5.
2UCl6+ 10HF → 2UF5 + 10HCl + Cl2
Synthesis
Uranium hexachloride can be synthesized from the reaction of uranium trioxide (UO3) with a mixture of liquid CCl4 and hot chlorine (Cl2). However the yield can be increased if the reaction carried out in the presence of UCl5. The UO3 is converted to UCl5, which in turn reacts with the excess Cl2 to form UCl6. It requires a substantial amount of heat for the reaction to take place; the temperature range is from 65oC to 170oC depending on the amount of reactants (ideal temp: 100oC - 125oC) The reaction is carried out in a closed gas-tight vessel (for example a glovebox) that can withstand the pressure that builds up.
Step 1: 2UO3 + 5Cl2 → 2UCl5 + 3O2
Step 2: 2UCl5 + Cl2 → 2UCl6
Overall reaction: 2UO3 + 6Cl2 → 2UCl6 + 3O2
This metal hexahalide can also be synthesized by blowing Cl2 gas over sublimed UCl4 at 350oC.
Step 1: 2UCl4 + Cl2 → 2UCl5
Step 2: 2UCl5 + Cl2 → 2UCl6
Overall Reaction: UCl4 + Cl2 → UCl6
