ATC code none PubChem CID 5610 Formula C8H11NO | CAS Number 51-67-2 IUPHAR/BPS 2150 Molar mass 137.179 g/mol | |
 | ||
Metabolism Metabolites | ||
Cheese the migraine diet
Tyramine (also spelled tyramin) (/ˈtaɪrəmin/ TY-rə-meen), also known by several other names, is a naturally occurring trace amine derived from the amino acid tyrosine. Tyramine acts as a catecholamine releasing agent. Notably, it is unable to cross the blood-brain barrier, resulting in only non-psychoactive peripheral sympathomimetic effects following ingestion. A hypertensive crisis can result, however, from ingestion of tyramine-rich foods in conjunction with monoamine oxidase inhibitors (MAOIs).
Contents
- Cheese the migraine diet
- Tyramine meaning
- Occurrence
- Physical effects and pharmacology
- Biosynthesis
- Chemistry
- United States
- Status in Florida
- Names
- References
Tyramine meaning
Occurrence
Tyramine occurs widely in plants and animals, and is metabolized by various enzymes, including monoamine oxidases. In foods, it often is produced by the decarboxylation of tyrosine during fermentation or decay. Foods containing considerable amounts of tyramine include meats that are potentially spoiled or pickled, aged, smoked, fermented, or marinated (some fish, poultry, and beef); most pork (except cured ham). Other foods containing considerable amounts of tyramine are chocolate; alcoholic beverages; and fermented foods, such as most cheeses (except ricotta, cottage, cream and Neufchâtel cheeses), sour cream, yogurt, shrimp paste, soy sauce, soybean condiments, teriyaki sauce, tempeh, miso soup, sauerkraut, kimchi, broad (fava) beans, green bean pods, Italian flat (Romano) beans, snow peas, edamame, avocados, bananas, pineapple, eggplants, figs, red plums, raspberries, peanuts, Brazil nuts, coconuts, processed meat, yeast, an array of cacti and the holiday plant mistletoe.
Physical effects and pharmacology
Evidence for the presence of tyramine in the human brain has been confirmed by postmortem analysis. Additionally, the possibility that tyramine acts directly as a neuromodulator was revealed by the discovery of a G protein-coupled receptor with high affinity for tyramine, called TAAR1. The TAAR1 receptor is found in the brain, as well as peripheral tissues, including the kidneys. Tyramine binds to both TAAR1 and TAAR2 as an agonist in humans.
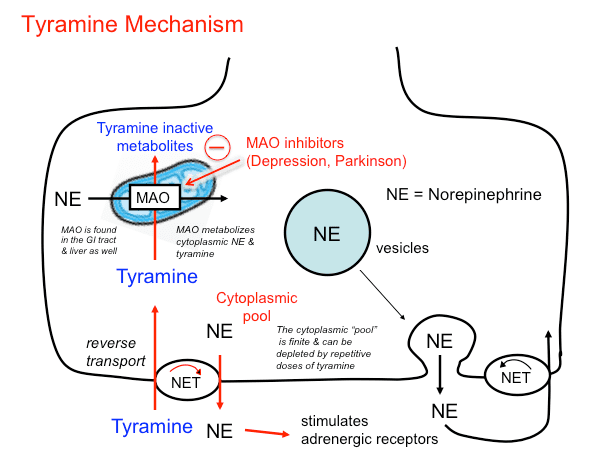
Tyramine is physiologically metabolized by monoamine oxidases (primarily MAO-A), FMO3, PNMT, DBH, and CYP2D6. In humans, if monoamine Metabolism is compromised by the use of monoamine oxidase inhibitors (MAOIs) and foods high in tyramine are ingested, a hypertensive crisis can result, as tyramine also can displace stored monoamines, such as dopamine, norepinephrine, and epinephrine, from pre-synaptic vesicles.
The first signs of this were discovered by a British pharmacist who noticed his wife, who at the time was on MAOI medication, had severe headaches when eating cheese. For this reason, the crisis is still called the "cheese effect" or "cheese crisis", though other foods can cause the same problem.
Most processed cheeses do not contain enough tyramine to cause hypertensive effects, although some aged cheeses (such as Stilton) do.
A large dietary intake of tyramine (or a dietary intake of tyramine while taking MAO inhibitors) can cause the tyramine pressor response, which is defined as an increase in systolic blood pressure of 30 mmHg or more. The displacement of norepinephrine (noradrenaline) from neuronal storage vesicles by acute tyramine ingestion is thought to cause the vasoconstriction and increased heart rate and blood pressure of the pressor response. In severe cases, adrenergic crisis can occur. Although the mechanism is unclear, tyramine ingestion also triggers migraines in sensitive individuals. Vasodilation, dopamine, and circulatory factors are all implicated in migraine. Double-blind trials suggest that the effects of tyramine on migraines may be adrenergic. Migraineurs are over-represented among those with inadequate natural monoamine oxidase, resulting in similar problems individuals taking MAO inhibitors. Many migraine triggers are high in tyramine.
If one has had repeated exposure to tyramine, however, there is a decreased pressor response; tyramine is degraded to octopamine, which is subsequently packaged in synaptic vesicles with norepinephrine (noradrenaline). Therefore, after repeated tyramine exposure, these vesicles contain an increased amount of octopamine and a relatively reduced amount of norepinephrine. When these vesicles are secreted upon tyramine ingestion, there is a decreased pressor response, as less norepinephrine is secreted into the synapse, and octopamine does not activate alpha or beta adrenergic receptors.
When using a MAO inhibitor (MAOI), the intake of approximately 10 to 25 mg of tyramine is required for a severe reaction compared to 6 to 10 mg for a mild reaction.
Research reveals a possible link between migraine and elevated levels of tyramine. A 2007 review published in Neurological Sciences presented data showing migraine and cluster headaches are characterised by an increase of circulating neurotransmitters and neuromodulators (including tyramine, octopamine and synephrine) in the hypothalamus, amygdala and dopaminergic system.
Biosynthesis
Biochemically, tyramine is produced by the decarboxylation of tyrosine via the action of the enzyme tyrosine decarboxylase. Tyramine can, in turn, be converted to methylated alkaloid derivatives N-methyltyramine, N,N-dimethyltyramine (hordenine), and N,N,N-trimethyltyramine (candicine).
In humans, tyramine is produced from tyrosine, as shown in the following diagram.
Chemistry
In the laboratory, tyramine can be synthesized in various ways, in particular by the decarboxylation of tyrosine.
United States
Tyramine is not scheduled at the federal level in the United States and is therefore legal to buy, sell, or possess.
Status in Florida
Tyramine is a Schedule I controlled substance, categorized as a hallucinogen, making it illegal to buy, sell, or possess in the state of Florida without a license at any purity level or any form whatever. The language in the Florida statute says tyramine is illegal in "any material, compound, mixture, or preparation that contains any quantity of [tyramine] or that contains any of [its] salts, isomers, including optical, positional, or geometric isomers, and salts of isomers, if the existence of such salts, isomers, and salts of isomers is possible within the specific chemical designation". This ban is likely the product of lawmakers overly eager to ban substituted phenethylamines, which tyramine is, in the mistaken belief that ring-substituted phenethylamines are hallucinogenic drugs like the 2C series of psychedelic substituted phenethylamines. The further banning of tyramine's optical isomers, positional isomers, or geometric isomers, and salts of isomers where they exist, means that meta-tyramine and phenylethanolamine, a substance found in every living human body, and other common, non-hallucinogenic substances are also illegal to buy, sell or possess in Florida. Given that tyramine occurs naturally in many foods and drinks (most commonly as a by-product of bacterial fermentation) e.g. wine, cheese, chocolate, Florida's total ban on the substance may prove difficult to enforce.
Names
Tyramine has also been called 4-hydroxyphenethylamine, para-tyramine, mydrial, or uteramin. The latter two names are not common. The IUPAC name is 4-(2-aminoethyl)phenol.
