Formula WO2Cl2 Density 4.67 g/cm³ | Molar mass 286.75 g/mol Appearance yellow crystals | |
 | ||
Tungsten dichloride dioxide is the chemical compound with the formula WO2Cl2. It is a yellow-colored solid. It is used as a precursor to other tungsten compounds. Like other tungsten halides, WO2Cl2 is sensitive to moisture, undergoing hydrolysis.
Contents
Preparation
WO2Cl2 is prepared by ligand redistribution reaction from tungsten trioxide and tungsten hexachloride:
2 WO3 + WCl6 → 3 WO2Cl2Using a two-zone tube furnace, a vacuum-sealed tube containing these solids is heated to 350 °C. The yellow product sublimes to the cooler end of the reaction tube. No redox occurs in this process. An alternative route highlights the oxophilicity of tungsten:
WCl6 + 2 O(Si(CH3)3)2 → 3 WO2Cl2 + 4 ClSi(CH3)3This reaction, like the preceding one, proceeds via the intermediacy of WOCl4.
Structure
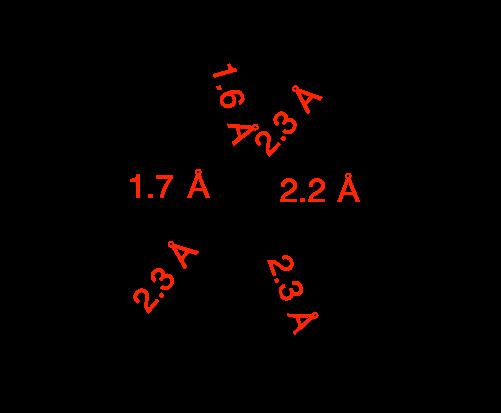
The compound is a polymer consisting of distorted octahedral W centres. The monomer is characterized by two short W-O distances, typical for a multiple W-O bond, and two long W-O distances more typical of a single or datice W-O bond.
Other tungsten oxy chlorides and related oxy halides
Tungsten forms a number of oxyhalides including WOCl4, WOCl3, WOCl2. The corresponding bromides (WOBr4, WOBr3, WOBr2) are also known as is WO2I2.
Reactions
WO2Cl2 is a Lewis acid, forming soluble adducts of the type WO2Cl2L2, where L is a donor ligand such as bipyridine and dimethoxyethane. Such complexes often cannot be prepared by depolymerization of the inorganic solid, but are generated in situ from WOCl4.
