EC number 2.3.2.13 ExPASy NiceZyme view | CAS number 80146-85-6 | |
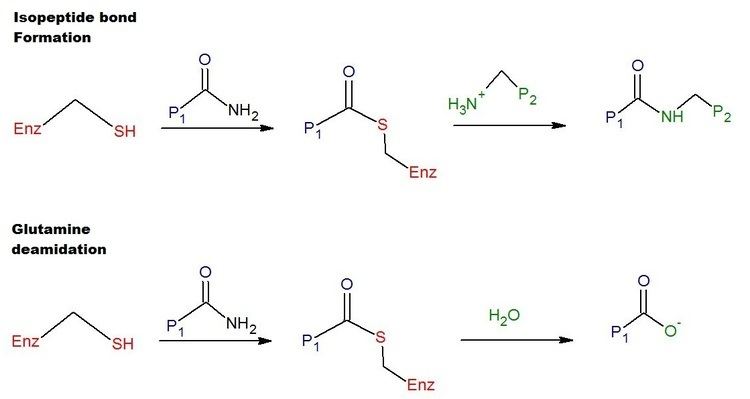 | ||
A transglutaminase is an enzyme that catalyzes the formation of an isopeptide bond between a free amine group (e.g., protein- or peptide-bound lysine) and the acyl group at the end of the side chain of protein- or peptide-bound glutamine. The reaction also produces a molecule of ammonia. Such an enzyme is classified as EC 2.3.2.13. Bonds formed by transglutaminase exhibit high resistance to proteolytic degradation (proteolysis).
Contents
- Physiological transglutaminases
- Mechanism of action
- Role in disease
- Industrial and culinary applications
- Molecular gastronomy
- References
Transglutaminases were first described in 1959. The exact biochemical activity of transglutaminases was discovered in blood coagulation protein factor XIII in 1968.
Physiological transglutaminases
Eight transglutaminases have been characterised.
Mechanism of action
Transglutaminases form extensively cross-linked, generally insoluble protein polymers. These biological polymers are indispensable for an organism to create barriers and stable structures. Examples are blood clots (coagulation factor XIII), as well as skin and hair.
The catalytic reaction is generally viewed as being irreversible, and must be closely monitored through extensive control mechanisms. A collection of the transglutaminase substrate proteins and interaction partners is accessible in the TRANSDAB database.
Role in disease
Deficiency of factor XIII (a rare genetic condition) predisposes to hemorrhage; concentrated enzyme can be used to correct the abnormality and reduce bleeding risk.
Anti-transglutaminase antibodies are found in celiac disease and may play a role in the small bowel damage in response to dietary gliadin that characterises this condition. In the related condition dermatitis herpetiformis, in which small bowel changes are often found and which responds to dietary exclusion of gliadin-containing wheat products, epidermal transglutaminase is the predominant autoantigen.
Recent research indicates that sufferers from neurological diseases like Huntington's and Parkinson's may have unusually high levels of one type of transglutaminase, tissue transglutaminase. It is hypothesized that tissue transglutaminase may be involved in the formation of the protein aggregates that causes Huntington's disease, although it is most likely not required.
Mutations in keratinocyte transglutaminase are implicated in lamellar ichthyosis.
Industrial and culinary applications
In commercial food processing, transglutaminase is used to bond proteins together. Examples of foods made using transglutaminase include imitation crabmeat, and fish balls. It is produced by Streptoverticillium mobaraense fermentation in commercial quantities or extracted from animal blood, and is used in a variety of processes, including the production of processed meat and fish products.
Transglutaminase can be used as a binding agent to improve the texture of protein-rich foods such as surimi or ham.
Molecular gastronomy
Transglutaminase is also used in molecular gastronomy to meld new textures with existing tastes. Besides these mainstream uses, transglutaminase has been used to create some unusual foods. British chef Heston Blumenthal is credited with the introduction of transglutaminase into modern cooking.
Wylie Dufresne, chef of New York's avant-garde restaurant wd~50, was introduced to transglutaminase by Blumenthal, and invented a "pasta" made from over 95% shrimp thanks to transglutaminase.
