Melting point 3,880 °C Density 14.3 g/cm³ Boiling point 4,780 °C | Formula TaCx Molar mass 192.96 g/mol Appearance Brown-gray powder | |
 | ||
Related refractory ceramic materials | ||
Tantalum carbides form a family of binary chemical compounds of tantalum and carbon with the empirical formula TaCx, where x usually varies between 0.4 and 1. They are extremely hard, brittle, refractory ceramic materials with metallic electrical conductivity. They appear as brown-gray powders, which are usually processed by sintering. Being important cermet materials, tantalum carbides are commercially used in tool bits for cutting applications and are sometimes added to tungsten carbide alloys. The melting points of tantalum carbides peak at about 3880 °C depending on the purity and measurement conditions; this value is among the highest for binary compounds. Only tantalum hafnium carbide may have a slightly higher melting point of about 3942 °C, whereas the melting point of hafnium carbide is comparable to that of TaC.
Contents

Preparation

TaCx powders of desired composition are prepared by heating a mixture of tantalum and graphite powders in vacuum or inert-gas atmosphere (argon). The heating is performed at temperature of about 2000 °C using a furnace or an arc-melting setup. An alternative technique is reduction of tantalum pentoxide by carbon in vacuum or hydrogen atmosphere at a temperature of 1500–1700 °C. This method was used to obtain tantalum carbide in 1876, but it lacks control over the stoichiometry of the product. Production of TaC directly from the elements has been reported through self-propagating high-temperature synthesis.
Crystal structure

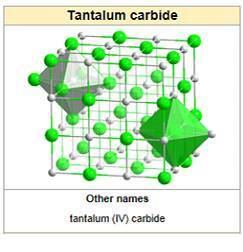
TaCx compounds have a cubic (rock-salt) crystal structure for x = 0.7–1.0; the lattice parameter increases with x. TaC0.5 has two major crystalline forms. The more stable one has an anti-cadmium iodide-type trigonal structure, which transforms upon heating to about 2000 °C into a hexagonal lattice with no long-range order for the carbon atoms.
Here Z is the number of formula units per unit cell, ρ is the density calculated from lattice parameters.
Properties
The bonding between tantalum and carbon atoms in tantalum carbides is a complex mixture of ionic, metallic and covalent contributions, and because of the strong covalent component, these carbides are very hard and brittle materials. For example, TaC has a microhardness of 1600–2000 kg/mm2 (~9 Mohs) and an elastic modulus of 285 GPa, whereas the corresponding values for tantalum are 110 kg/mm2 and 186 GPa. The hardness, yield stress and shear stress increase with the carbon content in TaCx. Tantalum carbides have metallic electrical conductivity, both in terms of its magnitude and temperature dependence. TaC is a superconductor with a relatively high transition temperature of TC = 10.35 K.

The magnetic properties of TaCx change from diamagnetic for x ≤ 0.9 to paramagnetic at larger x. An inverse behavior (para-diamagnetic transition with increasing x) is observed for HfCx, despite that it has the same crystal structure as TaCx.
Natural occurrence
Tantalcarbide is a natural form of tantalum carbide. It is cubic, extremely rare mineral.

