Entrez 6392 | Ensembl ENSG00000204370 | |
 | ||
Aliases SDHD, CBT1, CII-4, CWS3, PGL, PGL1, QPs3, SDH4, cybS, succinate dehydrogenase complex subunit D External IDs OMIM: 602690 MGI: 1914175 HomoloGene: 37718 GeneCards: SDHD | ||
Succinate dehydrogenase [ubiquinone] cytochrome b small subunit, mitochondrial (CybS), also known as succinate dehydrogenase complex subunit D (SDHD), is a protein that in humans is encoded by the SDHD gene. Names previously used for SDHD were PGL and PGL1. Succinate dehydrogenase is an important enzyme in both the citric acid cycle and the electron transport chain.
Contents
Structure
The SDHD gene is located on chromosome 11 at locus 11q23 and it spans 8,978 base pairs. The SDHD gene produces a 17 kDa protein composed of 159 amino acids.
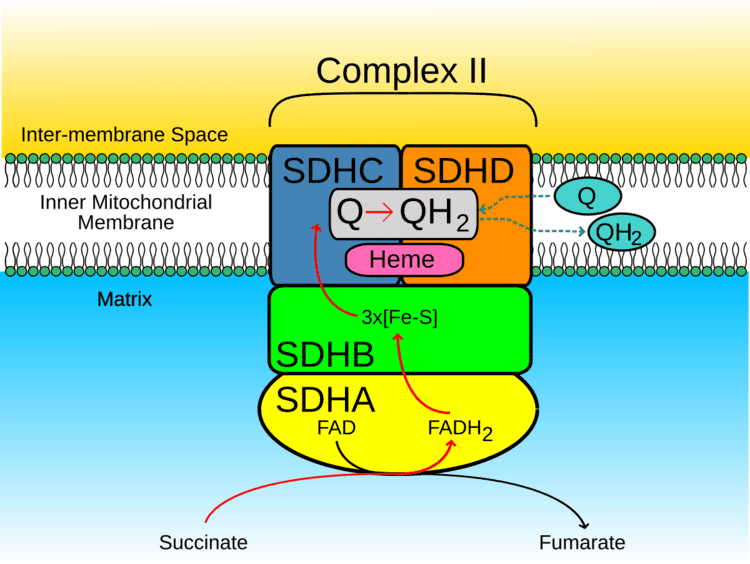
The SDHD protein is one of the two transmembrane subunits of the four-subunit succinate dehydrogenase (Complex II) protein complex that resides in the inner mitochondrial membrane. The other transmembrane subunit is SDHC. The SDHC/SDHD dimer is connected to the SDHB electron transport subunit which, in turn, is connected to the SDHA subunit.
Function
SDHD forms part of the transmembrane protein dimer with SDHC that anchors Complex II to the inner mitochondrial membrane. The SDHC/SDHD dimer provides binding sites for ubiquinone and water during electron transport at Complex II. Initially, SDHA oxidizes succinate via deprotonation at the FAD binding site, leaving fumarate, loosely bound to the active site, free to exit the protein. The electrons derived from succinate tunnel along the [Fe-S] relay in the SDHB subunit until they reach the [3Fe-4S] iron sulfur cluster. The electrons are then transferred to an awaiting ubiquinone molecule at the active site in the SDHC/SDHD dimer. The O1 carbonyl oxygen of ubiquinone is oriented at the active site (image 4) by hydrogen bond interactions with Tyr83 of SDHD. The presence of electrons in the [3Fe-4S] iron sulphur cluster induces the movement of ubiquinone into a second orientation. This facilitates a second hydrogen bond interaction between the O4 carbonyl group of ubiquinone and Ser27 of subunit C. Following the first single electron reduction step, a semiquinone radical species is formed. The second electron arrives from the [3Fe-4S] cluster to provide full reduction of the ubiquinone to ubiquinol.
Clinical significance
Mutations in the SDHD gene can cause familial paraganglioma.
Germline mutations in SDHD were first linked to hereditary paraganglioma in 2000. Since then, it has been shown that mutations in SDHB and to a lesser degree SDHC can cause paranglioma as well familial pheochromocytoma. Notably, the tumor spectrum is different for the different mutations. SDHB mutations often lead to metastatic disease that is extra-adrenal, while SDHD mutation related tumors are more typically benign, originating in the head and neck.
The exact mechanism for tumorigenesis is not determined, but it is suspected that malfunction of the SDH complex can cause a hypoxic response in the cell that leads to tumor formation. Mutations in the SDHB, SDHC, SDHD, and SDHAF2 genes lead to the loss or reduction of SDH enzyme activity. Because the mutated SDH enzyme cannot convert succinate to fumarate, succinate accumulates in the cell. As a result, the hypoxia pathways are triggered in normal oxygen conditions, which lead to abnormal cell growth and tumor formation. People living at higher altitudes (for example, the Andes mountains) are known to have an increased rate of benign paraganglioma, with the rate of disease increasing with the altitude of the population.
At least five variants in the SDHD gene have been identified in people with Cowden syndrome or a similar disorder called Cowden-like syndrome. These conditions are characterized by multiple tumor-like growths called hamartomas and an increased risk of developing certain cancers. When Cowden syndrome and Cowden-like syndrome are caused by SDHD gene mutations, the conditions are associated with a particularly high risk of developing breast and thyroid cancers. The SDHD gene variants associated with Cowden syndrome and Cowden-like syndrome change single amino acids in the SDHD protein, which likely alters the function of the SDH enzyme. Studies suggest that the defective enzyme could allow cells to grow and divide unchecked, leading to the formation of hamartomas and cancerous tumors. However, researchers are uncertain whether the identified SDHD gene variants are directly associated with Cowden syndrome and Cowden-like syndrome. Some of the variants described above have rarely been found in people without the features of these conditions.
Mutations in the SDHD gene have been found in a small number of people with Carney-Stratakis syndrome, a hereditary form of a cancer of the gastrointestinal tract called gastrointestinal stromal tumor (GIST). Those with Carney-Stratakis syndrome present with a noncancerous tumor associated with the nervous system called a paraganglioma or pheochromocytoma (a type of paraganglioma). An inherited SDHD gene mutation predisposes an individual to cancer formation. An additional mutation that deletes the normal copy of the gene is needed to cause Carney-Stratakis syndrome. This second mutation, called a somatic mutation, is acquired during a person's lifetime and is present only in tumor cells.
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles.
