 | ||
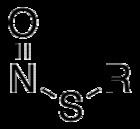
S-Nitrosothiols, also known as thionitrites, are organic compounds or functional groups containing a nitroso group attached to the sulfur atom of a thiol. S-Nitrosothiols have the general formula RSNO, where R denotes an organic group.
Contents
S-Nitrosothiols have received much attention in biochemistry because they serve as donors of the nitrosonium ion NO+, and nitric oxide and some organic nitroso derivatives serve as signaling molecules in living systems, especially related to vasodilation. Red blood cells, for instance, release S-nitrosothiols into the bloodstream under low-oxygen conditions, causing the blood vessels to dilate.
The addition of a nitroso group to a sulfur atom of an amino acid residue of some protein is known as S-nitrosylation or S-nitrosation. This is a reversible process and a major form of posttranslational modification of proteins.
S-Nitrosated proteins (SNOs) serve to transmit nitric oxide (NO) bioactivity and to regulate protein function through mechanisms analogous to phosphorylation: NO donors target specific amino acids motifs; post-translational modification leads to changes in protein activity, protein interactions, or subcellular location of target proteins; all major classes of proteins can undergo S-nitrosylation; and enzymes play a primary role in regulation of S-nitrosylation. Nitric oxide synthase (NOS) activity leads directly to SNO formation. NOSs are hemoproteins that combine reductase and oxygenase catalytic domains in one monomer to synthesize NO from the terminal nitrogen atom of L-arginine in the presence of NADPH and O2. NOSs target specific Cys residues for S-nitrosylation. Thiol S-nitrosylation and NO transfer reactions (transnitrosation reactions) are involved in virtually all classes of cell signaling, ranging from regulation of ion channels and G-protein coupled reactions to receptor stimulation and activation of nuclear regulatory protein.
Structure and reactions
The prefix "S" indicates that the NO group is attached to sulfur. The S-N-O angle deviates strongly from 180° because the nitrogen atom bears a lone pair of electrons.
S-Nitrosothiols arise from condensation from nitrous acid and a thiol:
RSH + HONO → RSNO + H2OMany other methods exist for their synthesis. They can be synthesized from thiols using NaNO2/H+, N2O3, N2O4, HNO, NOCl, RONO, NO2, HNO2, bovine aortic endothelial cells, among others. NaNO2/H+ and tert-butyl nitrite (tBuONO) are commonly used.
Once formed, these deeply colored compounds are often thermally unstable with respect to formation of the disulfide and nitric oxide:
2 RSNO → RSSR + 2 NOS-Nitrosothiols release NO+ upon treatment with acids:
RSNO + H+ → RSH + NO+and they can transfer nitroso groups to other thiols:
RSNO + R'SH → RSH + R'SNODetection
S-Nitrosothiols can be detected with UV-vis spectroscopy.
