Formula C6H15NO2S+ | Molar mass 164.247 g/mol | |
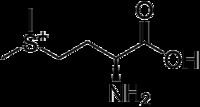 | ||
S-Methylmethionine (SMM) is a derivative of methionine with the chemical formula (CH3)2S+CH2CH2CH(NH3+)CO2−. This cation is an intermediate in many biosynthetic pathways owing to the sulfonium functional group. The natural derivative S-methylmethionine is biosynthesized from -methionine which is first converted to S-adenosylmethionine. The subsequent conversion, involving replacement of the adenosyl group by a methyl group is catalyzed by the enzyme methionine S-methyltransferase. S-methylmethionine is particularly abundant in plants, being more abundant than methionine.
Contents
S-Methylmethionine is sometimes referred to as vitamin U, but it is not considered a true vitamin. The term was coined in 1950 by Garnett Cheney for uncharacterized anti-ulcerogenic factors in raw cabbage juice that may help speed healing of peptic ulcers.
Biosynthesis and biochemical function
S-Methylmethionine arises via the methylation of methionine by S-adenosyl methionine (SAM). The coproduct is S-adenosyl homocysteine.
The biological roles of S-methylmethionine are not well understood. Speculated roles include methionine storage, use as a methyl donor, regulation of SAM. A few plants use S-methylmethionine as a precursor to the osmolyte dimethylsulfoniopropionate (DMSP). Intermediates include dimethylsulfoniumpropylamine and dimethylsulfoniumpropionaldehyde.
S-Methylmethionine is claimed to have protective effects in the gastrointestinal mucosa and in the liver.
Beer flavor precursor in barley malt
S-Methylmethionine is found in barley and during the malting process, particularly the curing stage in kilning, heat causes it to break down to form dimethyl sulfide (DMS).
