 | ||
Polyaspartic acid (PASA) is a biodegradable, water-soluble polyaminoacid. It is discussed as a possible replacement of many non-biodegradable polymers. It is used as a homopolymer and in various copolymers. In nature, PASA has been found in as fragments of larger proteins with length up to 50 amino acids, but as of 2004 had not been isolated as a pure homo polymeric material from any natural source. The first isolation of synthetic oligomeric sodium polyaspartate, obtained by thermal polycondensation of aspartic acid, was reported by Hugo Schiff in late 19th century. Later it was proposed that thermal polymerization process leads through polysuccinimide intermediate. Polyaspartic acid is produced industrially in both the acid form and as the sodium polyaspartate salt.
Contents
Properties and structure
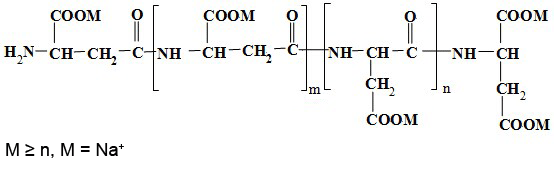
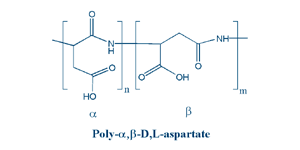
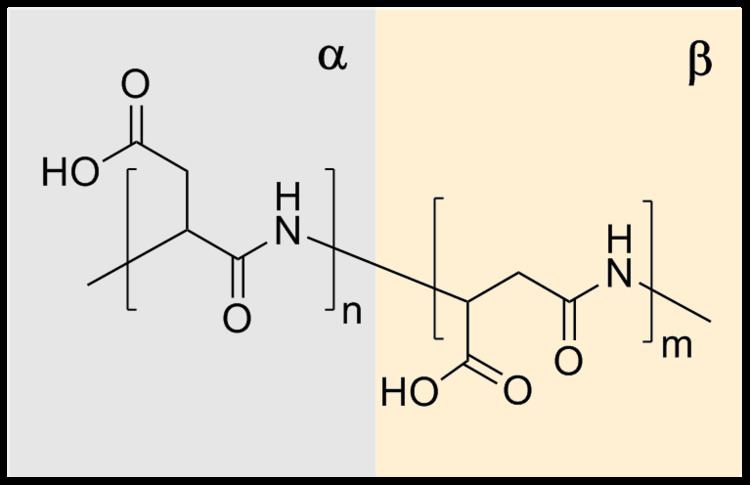
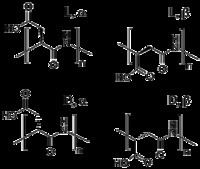
Due to presence of carboxylic groups it is polyelectrolyte with anionic character. Naturally occurring PASA fragments consists of α,-linked L-aspartatic acid. In contrast, the repeating unit of synthetic polyaspartic acid may exist in four isomeric forms, depending on the stereochemistry of starting material (D- and L-aspartic acid) and synthetic procedure leading to α and β links.
Synthesis
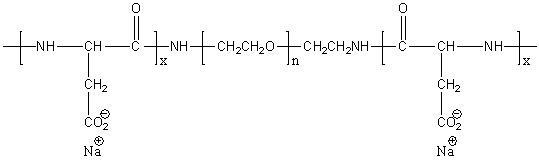
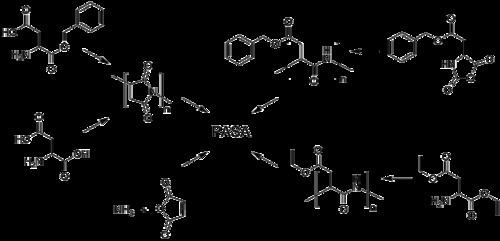
Many different routes lead to PASA. In the simplest and the oldest approach aspartic acid is heated to induce dehydration. In a subsequent step the resulting polysuccinimide is treated with aqueous sodium hydroxide, which yields partial opening of the succinimide rings. In this process sodium-DL-(α,β)-poly(aspartate) with 30% α-linkages and 70% β-linkages randomly distributed along the polymer chain, and racemized chiral center of aspartic acid is produced. There were many catalysts reported for improving thermal polymerization method. Main benefits from their application is increasing of the conversion rate and higher molecular weight of the product. Polyaspartic acid can also be synthesized by polymerization of maleic anhydride in presence of ammonium hydroxide. High control over repeating unit isomers can be achieved by polymerization of N-carboxyanhydride (NCA) derivatives, by polymerization of aspartic acid esters or by application of enzyme catalyzed reaction. Pure homopolymers, D- or L-PASA with α- or β-links only, can be synthesized using those methods.
Applications
Polyaspartic acid and its derivatives are biodegradable alternatives to traditional polyanionic materials, in particular polyacrylic acid. PASA has ability to inhibit deposition of calcium carbonate, calcium sulfate, barium sulfate, and calcium phosphate and can be used as an antiscalining agent in cooling water systems, water desalination processes, and waste water treatment operations. In addition and due to its ability to chelate metal ions, it provides corrosion inhibition. It can act as a super-swelling material in diapers, feminine hygiene products, and food packaging. It can also be used as biodegradable detergent and dispersant for various applications.