Entrez 5479 | Ensembl ENSG00000166794 | |
 | ||
Aliases PPIB, CYP-S1, CYPB, HEL-S-39, OI9, SCYLP, peptidylprolyl isomerase B External IDs MGI: 97750 HomoloGene: 726 GeneCards: PPIB | ||
Kursus ppib 23 24 april 2015
Peptidyl-prolyl cis-trans isomerase B is an enzyme that in humans is encoded by the PPIB gene. As a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family, this protein catalyzes the cis-trans isomerization of proline imidic peptide bonds, which allows it to regulate protein folding of type I collagen. PPIB localizes to the endoplasmic reticulum (ER) and participates in many biological processes, including mitochondrial metabolism, apoptosis, redox, and inflammation, as well as in related diseases and conditions, such as ischemic reperfusion injury, AIDS, and cancer. It is also associated with viral infections.
Contents
- Kursus ppib 23 24 april 2015
- Two little hands to clap clap clap 3d animation english nursery rhymes for children
- Structure
- Function
- Clinical significance
- Interactions
- References
Two little hands to clap clap clap 3d animation english nursery rhymes for children
Structure
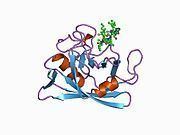
Like other cyclophilins, PPIB forms a β-barrel structure with a hydrophobic core. This β-barrel is composed of eight anti-parallel β-strands and capped by two α-helices at the top and bottom. In addition, the β-turns and loops in the strands contribute to the flexibility of the barrel. In particular, PPIB is a 21 kDa protein which contains a C-terminal ER retention motif that directs the protein to the ER organelle, while its N-terminal extension attaches it to its substrates.
Function
PPIB is a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family. PPIases catalyze the cis-trans isomerization of proline imidic peptide bonds and regulate protein folding and maturation. Generally, PPIases are found in all eubacteria and eukaryotes, as well as in a few archaebacteria, and thus are highly conserved. PPIB is the second of 18 cyclophilins to be identified in humans, after CypA. The PPIase family is further divided into three structurally distinct subfamilies: cyclophilin (CyP), FK506-binding protein (FKBP), and parvulin (Pvn). As a cyclophilin, PPIB binds cyclosporin A (CsA) and can be found within in the cell or secreted by the cell. In eukaryotes, cyclophilins localize ubiquitously to many cell and tissue types. In addition to PPIase and protein chaperone activities, cyclophilins function in mitochondrial metabolism, apoptosis, immunological response, inflammation, and cell growth and proliferation. Along with PPIC, PPIB localizes to the endoplasmic reticulum (ER), where it maintains redox homeostasis. Depletion of these two cyclophilins leads to hyperoxidation of the ER.
In the ER, PPIB interacts with proteins such as P3H1, CRTAP, BiP, GRP94, PDI, and calreticulin to form foldase and chaperone complexes and facilitate protein folding, especially for type I collagen. This protein is the major PPIase for type I collagen, since the collagen contains an abundance of prolines that require cis-trans isomerization for proper folding. Thus, PPIB is essential for collagen biosynthesis and post-translational modification and affects fibril assembly, matrix cross-linking, and bone mineralization.
In addition, it is associated with the secretory pathway and released in biological fluids. This protein can bind to cells derived from T- and B-lymphocytes, and may regulate cyclosporine A-mediated immunosuppression. In one experiment, the addition of PPIB into cell cultures in vitro induced chemotaxis and integrin-mediated adhesion of T cells to the extracellular matrix (ECM), suggesting that it might function in innate immunity by recruing T cells into infected tissue in vivo.
Clinical significance
As a cyclophilin, PPIB binds the immunosuppressive drug CsA to form a CsA-cyclophilin complex, which then targets calcineurin to inhibit the signaling pathway for T-cell activation.
In cardiac myogenic cells, cyclophilins have been observed to be activated by heat shock and hypoxia-reoxygenation as well as complex with heat shock proteins. Thus, cyclophilins may function in cardioprotection during ischemia-reperfusion injury.
PPIB contributes to the replication and infection of viruses causing diseases such as AIDS, hepatitis C, measles, and influenza A. Thus, therapeutic targeting of PPIB with selective inhibitors may prove effective in combating viral infections and inflammation. Currently, PPIB is employed as a biomarker for various types of cancer. Moreover, there are two antigenic epitopes (CypB84-92 and CypB91-99) recognized by HLA-A24-restricted and tumor-specific cytotoxic T lymphocytes which could be used as cancer vaccines, and in fact, were used to treat lung cancer in a clinical trial.
Interactions
PPIB has been shown to interact with:
