Formula C4H8OS | Appearance Colorless liquid | |
 | ||
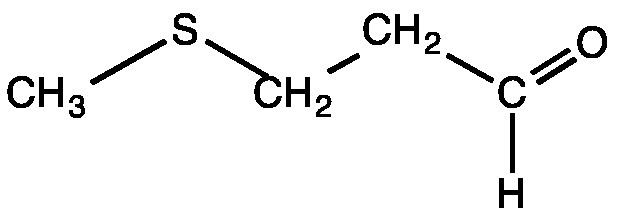
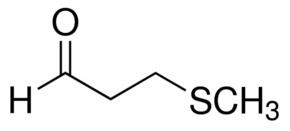
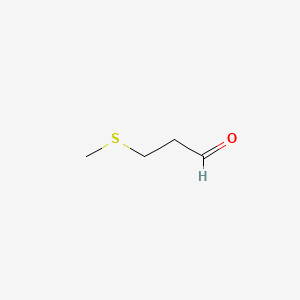
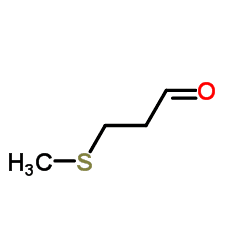
Methional is an organic compound with the formula CH3SCH2CH2CHO. It is a colorless liquid that is a degradation product of methionine. It is a notable flavor in potato-based snacks, namely potato chips, one of the most popular foods containing methional. Traces of the compound can also be found in black tea and green tea based products. Methional contains both aldehyde and thioether functional groups. It is readily soluble in alcohol solvents, including propylene glycol and dipropylene glycol.
Contents
Occurrence
In nature, methional is a thermally-induced volatile flavor compound. For instance, the heat-initiated Maillard reaction of reducing sugars and amino acids forms the initial basis of methional's composition. The formation of methional stems from the interaction of α-dicarbonyl compounds (intermediate products in the Maillard reaction) with methionine (Met) by the Strecker degradation reaction:
CH3SCH2CH2(NH2)CHCO2H + O → CH3SCH2CH2(HN=)CCO2H + H2OCH3SCH2CH2(HN=)CCO2H + H2O → CH3SCH2CH2CHO + NH3 + CO2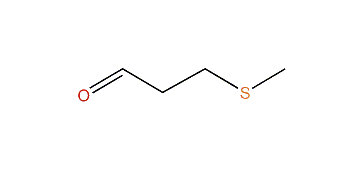
Methional easily degrades into methanethiol, which then oxidizes into dimethyl disulfide. Dimethyl disulfide is partly responsible for the "reactive sulfur" that causes the overall taste of potatoes. Furthermore, the methionine resulting from the Strecker degradation reaction produces alkyl pyrazines, which contribute to the flavors in roasted, toasted, or thermally processed foods. Due to the ease of its decomposition, a large portion of methional is lost during potato processing.
Similarly, in the presence of flavin mononucleotide (FMN) and light, methionine is nonenzymatically oxidized into methional, ammonia, and carbon dioxide.
CH3SCH2CH2(NH2)CHCO2H → CH3SCH2CH2CHO + NH3 + CO2Preparation
In the laboratory, methional is prepared by the Stephen aldehyde synthesis. Accordingly, 3-methylmercatopropionitrile is reduced to the iminium salt with stannous chloride, which is subsequently hydrolyzed as represented by these idealized reactions<!idealized because the SnCl4 gets wrecked in the final step, BTW, first reaction is even questionable>
CH3S(CH2)2CN + SnCl2 + 2 HCl → [CH3S(CH2)2CHNH2Cl]+ [SnCl3]−[CH3S(CH2)2CHNH2Cl]+ [SnCl3]− + H2O → CH3S(CH2)2CHO + NH3 + SnCl4Another methional synthesis involves the reaction of methanethiol and acrolein.
CH3SH + CH2=CHCHO → CH3SCH2CH2CHOBiological routes
Because of their high cost, methional or its precursor methionine are not added during potato processing. In order to intensify flavoring of heat-processed potato foods, biotechnological approaches are used to increase methionine levels, and thus methional levels, in potato foods.
Biologically, the enzyme aminotransferase acts on methionine to remove the amine and form an α-keto-γ-methylthiobutyric acid. As catalyzed by α-keto acid decarboxylase, this ketomethylthiobutyric acid converts to methional.
CH3S(CH2)2CH(NH2)CO2H + O → CH3S(CH2)2COCO2H + NH3CH3S(CH2)2COCO2H → CH3S(CH2)2CHO + CO2