Formula C4H6O4Hg Density 3.27 g/cm³ Appearance white-yellow crystals | Molar mass 318.7 g/mol Melting point 179 °C | |
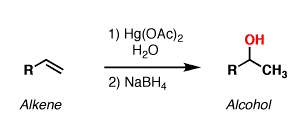 | ||
Mercury(II) acetate is the chemical compound with the formula Hg(O2CCH3)2. Commonly abbreviated Hg(OAc)2, this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors.
Contents
Structure
Mercury(II) acetate is a crystalline solid consisting of isolated Hg(OAc)2 molecules with Hg-O distances of 2.07 Å. Three long, weak intermolecular Hg···O bonds of about 2.75 Å are also present, resulting in a slightly distorted square pyramidal coordination geometry at Hg.
Reactions
Arenes undergo "mercuration" upon treatment with Hg(OAc)2. The one acetate group that remains on mercury can be displaced by chloride:
C6H5OH + Hg(OAc)2 → C6H4(OH)-2-HgOAc + HOAcC6H4(OH)-2-HgOAc + NaCl → C6H4(OH)-2-HgCl + NaOAcThe Hg2+ center binds to alkenes, inducing the addition of hydroxide and alkoxide. For example, treatment of methylacrylate with mercuric acetate in methanol gives an α-mercuri ester:
Hg(OAc)2 + CH2=CHCO2CH3 + CH3OH → CH3OCH2CH(HgOAc)CO2CH3 + HOAcMercury(II) has a high affinity for sulfur ligands. Hg(OAc)2 can be used as a reagent to remove the acetamidomethyl protecting group, which is used to "protect" thiol groups in organic synthesis. Similarly Hg(OAc)2 is a standard reagent to convert thiocarbonate esters into dithiocarbonates:
(RS)2C=S + H2O + Hg(OAc)2 → (RS)2C=O + HgS + 2 HOAcMercury(II) acetate is used for oxymercuration reactions.
