Formula MgF2 Molar mass 62.3018 g/mol Density 3.15 g/cm³ | Melting point 1,263 °C Boiling point 2,260 °C Appearance white tetragonal crystals | |
 | ||
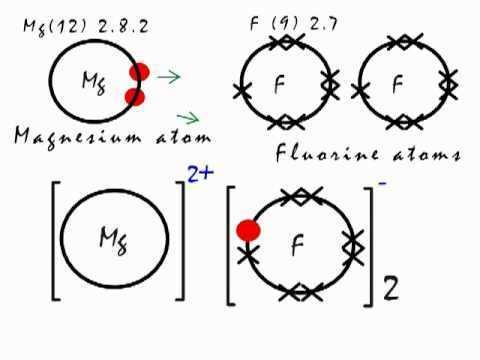
Dot cross diagram magnesium fluoride
Magnesium fluoride is an inorganic compound with the formula MgF2. The compound is a white crystalline salt and is transparent over a wide range of wavelengths, with commercial uses in optics that are also used in space telescopes. It occurs naturally as the rare mineral sellaite.
Contents
- Dot cross diagram magnesium fluoride
- This is how the ionic bond forms in magnesium fluoride mgf2
- Production and structure
- Optics
- References
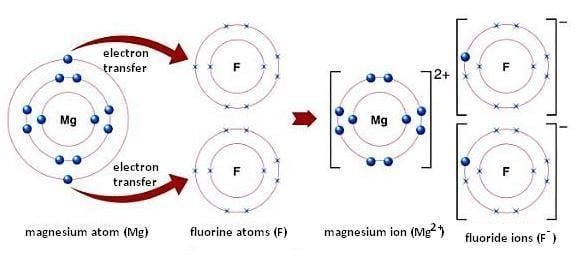
This is how the ionic bond forms in magnesium fluoride mgf2
Production and structure
Magnesium fluoride is prepared from magnesium oxide with sources of hydrogen fluoride such as ammonium bifluoride:
MgO + (NH4)HF2 → MgF2 + NH3 + H2ORelated metathesis reactions are also feasible.

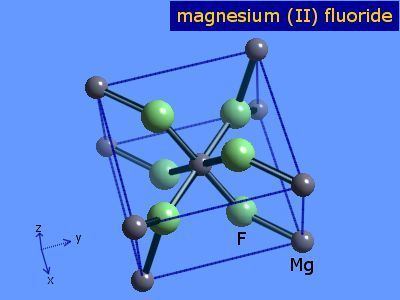
The compound crystallizes as tetragonal birefringent crystals. The structure of the compound is similar to that in rutile, featuring octahedral Mg2+ centers and 3-coordinate fluoride centres.
Optics

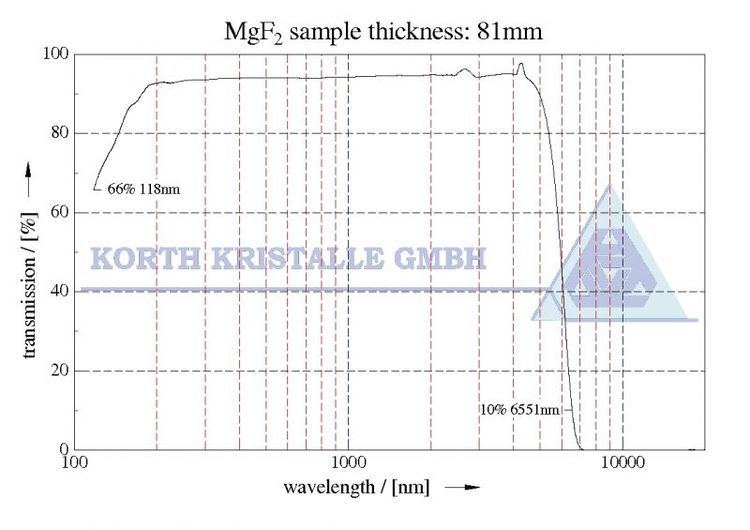
Magnesium fluoride is transparent over an extremely wide range of wavelengths. Windows, lenses, and prisms made of this material can be used over the entire range of wavelengths from 0.120 μm (vacuum ultraviolet) to 8.0 μm (infrared). High quality synthetic VUV grade MgF2 is quite expensive, in the region of $3/kg (2007) but the real cost of optics in this material is due to relatively low volume manufacture. However, with lithium fluoride it is one of the two materials that will transmit in the vacuum ultraviolet range at 121 nm (Lyman alpha) and this is where it finds its application. Lower grade MgF2 is sometimes used in the infrared but here it is inferior to calcium fluoride. MgF2 is tough and works and polishes well, but it is slightly birefringent and should be cut with the optic axis perpendicular to the plane of the window or lens.
Due to its having a suitable refractive index of 1.37, thin layers of MgF2 are very commonly used on the surfaces of optical elements as inexpensive anti-reflective coatings.
The Verdet constant of (MgF2) at 632.8 nm is 0.00810 arcmin G−1 cm−1.