 | ||
MOSCED (short for “Modified Separation of Cohesive Energy Density Model”) is a thermodynamic model for the estimation of limiting activity coefficients (also known as activity coefficient at infinite dilution). From a historical point of view MOSCED can be regarded as an improved modification of the Hansen method and the Hildebrand solubility model. The first publication is from 1984 and a major revision of parameters has been done 2005. This revised version is described here.
Contents
Basic principle
MOSCED uses component-specific parameters describing electronic properties of a compound. These five properties are partly derived from experimental values and partly fitted to experimental data. In addition to the five electronic properties the model uses the molar volume for every component.
These parameters are then entered in several equations to obtain the limiting activity coefficient of an infinitely diluted solute in a solvent. These equations have further parameters which have been found empirically.
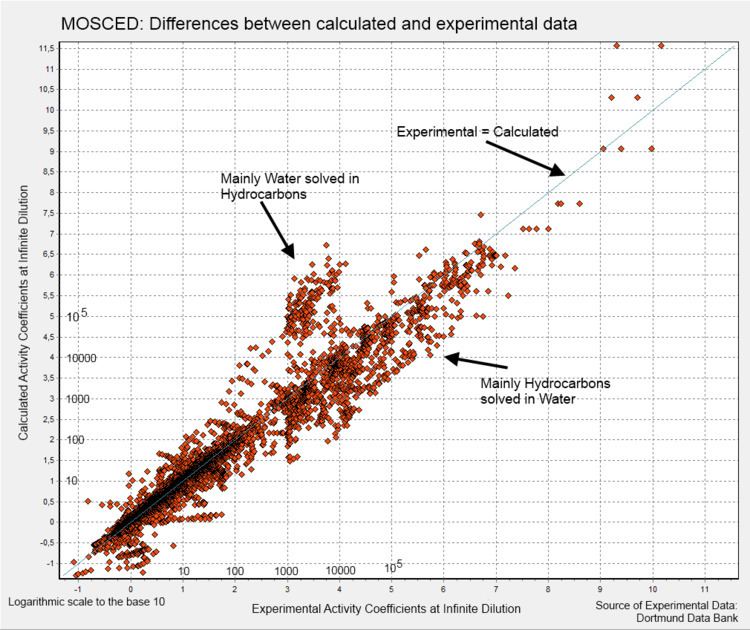
The authors found an average absolute deviation of 10.6% against their database of experimental data. The database contains limiting activity coefficients of binary systems of non-polar, polar and hydrogen compounds, but no water. As can be seen in the deviation chart, the systems with water deviate significantly.
Equations
with
Important note: The value 3.4 in the equation for ξ is different from the value 3.24 in the original publication. The 3.24 has been verified to be a typing error.
The activity coefficient of the solute and solvent can be extended to other concentrations by applying the principle of the Margules equation. This gives:
where
is the volume fraction and
Model parameters
The model uses five component specific properties to characterize the interaction forces between a solute and its solvent. Some of these properties are derived from other known component properties and some are fitted to experimental data obtained from data banks.
Liquid molar volume
The molar liquid volume ν is given in cm³/mol and assumed to be temperature-independent.
Dispersion parameter
The dispersion parameter λ describes the polarizability of a molecule.
Polarity parameter
The polarity parameter τ describes the fixed dipole of a molecule.
Induction parameter
The induction parameter q describes the effects of induced dipoles (induced by fixed dipoles). For structures with an aromatic ring the value is set to 0.9, for aliphatic rings and chains this value is set on 1. For some compounds the q-parameter is optimized between 0.9 and 1 (e.g. hexene, octene).
Acidity and basicity parameters
These parameters describe the effects of hydrogen-bonding during solving and association.
