MedlinePlus a685040 Molar mass 1,209.4 g/mol | Pregnancycategory X ATC code L02AE02 (WHO) CAS ID 53714-56-0 | |
 | ||
Trade names Lupron, Eligard, others AHFS/Drugs.com Consumer Drug Information | ||
Nurse linda ivf medication injections lupron
Leuprorelin, also known as leuprolide, is a manufactured version of a hormone used to treat prostate cancer, breast cancer, endometriosis, uterine fibroids, and early puberty. It is given by injection into a muscle or under the skin.
Contents
- Nurse linda ivf medication injections lupron
- Medical uses
- Adverse effects
- Mechanism of action
- Chemistry
- Approvals
- Lupron protocol
- Veterinary use
- Research
- References
Common side effects include hot flashes, unstable mood, trouble sleeping, headaches, and pain at the site of injection. Other side effects may include high blood sugar, allergic reactions, and problems with the pituitary gland. Use during pregnancy may harm the baby. Leuprorelin is in the gonadotropin-releasing hormone (GnRH) analogue family of medication. It works by decreasing gonadotropin and therefore decreasing testosterone and estradiol.
Leuprorelin was approved for medical use in the United States in 1985. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. In the United Kingdom a monthly dose costs the NHS about 75.24 pounds. In the United States the equivalent dose has a wholesale cost of 1,011.93 USD. It is sold under the brand name Lupron among others.
Medical uses
Leuprorelin may be used in the treatment of hormone-responsive cancers such as prostate cancer and breast cancer. It may also be used for estrogen-dependent conditions such as endometriosis or uterine fibroids.
It may be used for precocious puberty in both males and females, and to prevent premature ovulation in cycles of controlled ovarian stimulation for in vitro fertilization (IVF).
Leuprorelin, along with triptorelin and goserelin, are often used to delay puberty in transgender youth until they are old enough to begin hormone replacement therapy. They are also sometimes used as superior alternatives to anti-androgens like spironolactone and cyproterone acetate for suppressing testosterone production in trans women.
It is considered a possible treatment for paraphilias. Leuprorelin has been tested as a treatment for reducing sexual urges in pedophiles and other cases of paraphilia.
Adverse effects
Common side effects of Lupron Injection include redness/burning/stinging/pain/bruising at the injection site, hot flashes (flushing), increased sweating, night sweats, tiredness, headache, upset stomach, nausea, diarrhea, constipation, stomach pain, breast swelling or tenderness, acne, joint/muscle aches or pain, trouble sleeping (insomnia), reduced sexual interest, vaginal discomfort/dryness/itching/discharge, vaginal bleeding, swelling of the ankles/feet, increased urination at night, dizziness, breakthrough bleeding in a female child during the first 2 months of leuprorelin treatment, weakness, chills, clammy skin, skin redness, itching, or scaling, testicle pain, impotence, depression, or memory problems.
Mechanism of action
Leuprorelin acts as an agonist at pituitary GnRH receptors. By interrupting the normal pulsatile stimulation of, and thus desensitizing, the GnRH receptors, it indirectly downregulates the secretion of gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH), leading to hypogonadism and thus a dramatic reduction in estradiol and testosterone levels in both sexes.
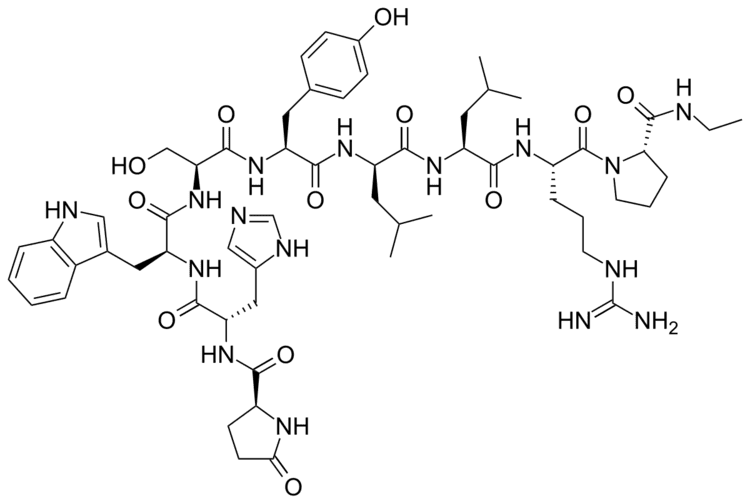
Chemistry
The peptide sequence is Pyr-His-Trp-Ser-Tyr-D-Leu-Leu-Arg-Pro-NHEt (Pyr = L-Pyroglutamyl).
Approvals
Leuprorelin is marketed by Bayer AG under the brand name Viadur, by Tolmar under the brand name Eligard, and by TAP Pharmaceuticals (1985–2008), by Varian Darou Pajooh under the brand name Leupromer and Abbott Laboratories (2008-current) under the brand name Lupron. It is available as a slow-release implant or subcutaneous/intramuscular injection.
In the UK and Ireland, leuprorelin is marketed by Takeda UK as Prostap SR (one-month injection) and Prostap 3 (three-month injection).
"Lupron protocol"
A 2005 paper suggested leuprorelin as a possible treatment for autism, the hypothetical method of action being the now defunct hypothesis that autism is caused by mercury, with the additional unfounded assumption that mercury binds irreversibly to testosterone and therefore leuprorelin can help cure autism by lowering the testosterone levels and thereby mercury levels. However there is no scientifically valid or reliable research to show its effectiveness in treating autism. This use has been termed the "Lupron protocol" and Mark Geier, the proponent of the hypothesis, has frequently been barred from testifying in vaccine-autism related cases on the grounds of not being sufficiently expert in that particular issue and has had his medical license revoked. Medical experts have referred to Geier's claims as "junk science".
Veterinary use
Leuprorelin has been used in two cases of ferrets with chronic adrenal disease, one with primary hyperaldosteronism, and one with hyperadrenocorticism
Research
As of 2006 leuprorelin was under investigation for possible use in the treatment of mild to moderate Alzheimer's disease.
