Pronunciation /kᵻˈtoʊsᵻs/ DiseasesDB 29485 | ICD-9-CM 276.2 MeSH D007662 | |
 | ||
Specialty Endocrinology specialty | ||
Ketosis is a metabolic state in which some of the body's energy supply comes from ketone bodies in the blood, in contrast to a state of glycolysis in which blood glucose provides most of the energy.
Contents
- Ketoacidosis
- Diet
- Mechanism
- Diagnosis
- Severity
- Controversy
- Inuit People
- Adaptation
- Veterinary medicine
- References
Ketosis is a nutritional process characterised by serum concentrations of ketone bodies over 0.5 mM, with low and stable levels of insulin and blood glucose. It is almost always generalized with hyperketonemia, that is, an elevated level of ketone bodies in the blood throughout the body. Ketone bodies are formed by ketogenesis when liver glycogen stores are depleted (or from metabolising medium-chain triglycerides). The main ketone bodies used for energy are acetoacetate and β-hydroxybutyrate, and the levels of ketone bodies are regulated mainly by insulin and glucagon. Most cells in the body can use both glucose and ketone bodies for fuel, and during ketosis, free fatty acids and glucose synthesis (gluconeogenesis) fuel the remainder.
Longer-term ketosis may result from fasting or staying on a low-carbohydrate diet, and deliberately induced ketosis serves as a medical intervention for various conditions, such as intractable epilepsy, and the various types of diabetes. In glycolysis, higher levels of insulin promote storage of body fat and block release of fat from adipose tissues, while in ketosis, fat reserves are readily released and consumed. For this reason, ketosis is sometimes referred to as the body's "fat burning" mode.
While ketosis and ketoacidosis are frequently confused with one another, they are certainly not the same. Ketoacidosis is an acute life-threatening condition requiring prompt medical intervention. However, there are situations (such as treatment-resistant epilepsy) where ketosis can be rather beneficial to health.
Ketoacidosis
Ketone bodies are acidic, but acid-base homeostasis in the blood is normally maintained through bicarbonate buffering, respiratory compensation to vary the amount of CO2 in the bloodstream, hydrogen ion absorption by tissue proteins and bone, and renal compensation through increased excretion of dihydrogen phosphate and ammonium ions. Prolonged excess of ketone bodies can overwhelm normal compensatory mechanisms, leading to acidosis if blood pH falls below 7.35.
There are two major causes of ketoacidosis:
A mild acidosis may result from prolonged fasting or when following a ketogenic diet or a very low calorie diet.
Diet
If the diet is changed from one that is high in carbohydrates to one that does not provide sufficient carbohydrate to replenish glycogen stores, the body goes through a set of stages to enter ketosis. During the initial stages of this process, blood glucose levels are maintained through gluconeogenesis, and the adult brain does not burn ketones. However, the brain makes immediate use of ketones for lipid synthesis in the brain. After about 48 hours of this process, the brain starts burning ketones in order to more directly use the energy from the fat stores that are being depended upon, and to reserve the glucose only for its absolute needs, thus avoiding the depletion of the body's protein store in the muscles.
Ketosis is deliberately induced by use of a ketogenic diet as a medical intervention in cases of intractable epilepsy. Other uses of low-carbohydrate diets remain controversial. Induced ketosis or low-carbohydrate diet terms have very wide interpretation. Therefore, Stephen S. Phinney and Jeff S. Volek coined the term "nutritional ketosis" to avoid the confusion.
Carbohydrate deprivation to the point of ketosis has been argued to have both negative and positive effects on health.
Mechanism
Fats stored in adipose tissue are released from the fat cells into the blood as free fatty acids and glycerol when insulin levels are low and glucagon and epinephrine levels in the blood are high. This occurs between meals, during fasting, starvation and strenuous exercise, when blood glucose levels are likely to fall. Fatty acids are very high energy fuels, and are taken up by all metabolizing cells which have mitochondria. Fatty acids can only be metabolized in the mitochondria. Red blood cells do not contain mitochondria and are therefore entirely dependent on glycolysis for their energy requirements. In all other tissues the fatty acids that enter the metabolizing cells are combined with co-enzyme A to form acyl-CoA chains. These are transferred into the mitochondria of the cells, where they are broken down into acetyl-CoA units by a sequence of reactions known as β-oxidation.
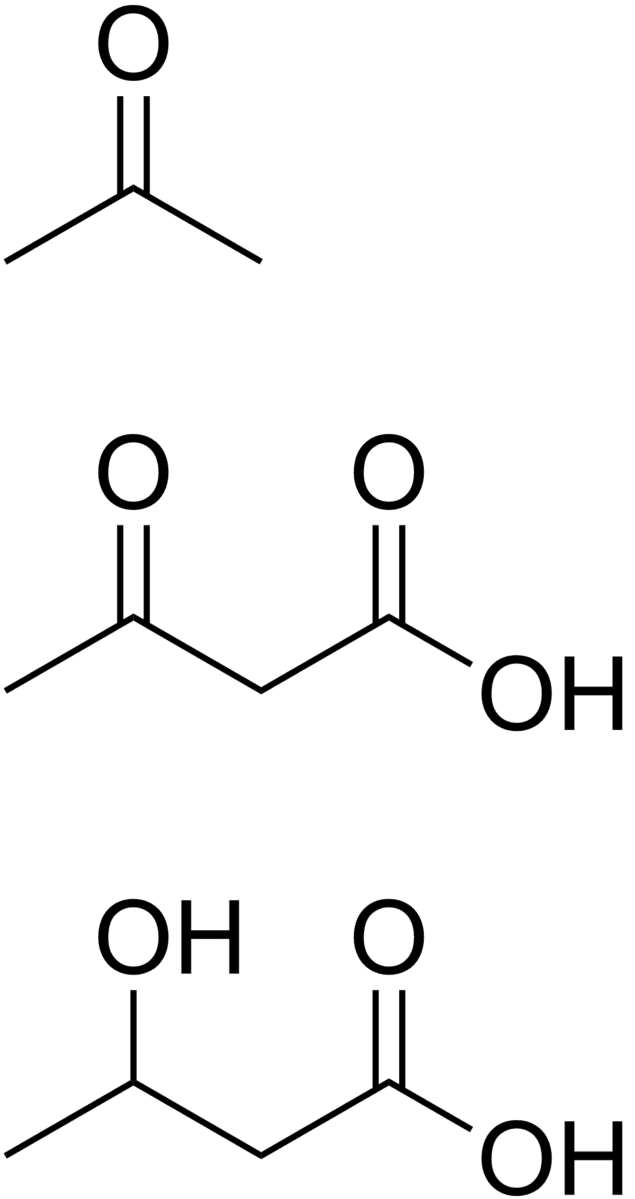
The acetyl-CoA produced by β-oxidation enters the citric acid cycle in the mitochondrion by combining with oxaloacetate to form citrate. This results in the complete combustion of the acetyl-CoA to CO2 and water. The energy released in this process is captured in the form of 1 GTP and 11 ATP molecules per acetyl-CoA molecule oxidized. This is the fate of acetyl-CoA wherever β-oxidation of fatty acids occurs, except under certain circumstances in the liver. In the liver oxaloacetate is wholly or partially diverted into the gluconeogenic pathway during fasting, starvation, a low carbohydrate diet, prolonged strenuous exercise, and in uncontrolled type 1 diabetes mellitus. Under these circumstances oxaloacetate is hydrogenated to malate which is removed from the mitochondrion to be converted into glucose in the cytoplasm of the liver cells, from where it is released into the blood. In the liver, therefore, oxaloacetate is unavailable for condensation with acetyl-CoA when significant gluconeogenesis has been stimulated by low (or absent) insulin and high glucagon concentrations in the blood. Under these circumstances acetyl-CoA is diverted to the formation of acetoacetate and beta-hydroxybutyrate. Acetoacetate, beta-hydroxybutyrate, and their spontaneous breakdown product, acetone, are frequently, but confusingly, known as ketone bodies (as they are not "bodies" at all, but water-soluble chemical substances). The ketone bodies are released by the liver into the blood. All cells with mitochondria can take ketone bodies up from the blood and reconvert them into acetyl-CoA, which can then be used as fuel in their citric acid cycles, as no other tissue can divert its oxaloacetate into the gluconeogenic pathway in the way that the liver does this. Unlike free fatty acids, ketone bodies can cross the blood-brain barrier and are therefore available as fuel for the cells of the central nervous system, acting as a substitute for glucose, on which these cells normally survive. The occurrence of high levels of ketone bodies in the blood during starvation, a low carbohydrate diet, prolonged heavy exercise and uncontrolled type 1 diabetes mellitus is known as ketosis, and in its extreme form in out-of-control diabetes mellitus, as ketoacidosis.
Diagnosis
Whether ketosis is taking place can be checked by using special urine test strips such as Ketostix. The strips have a small pad on the end which is dipped in a fresh specimen of urine. Within a matter of seconds, the strip changes color indicating the level of acetoacetate ketone bodies detected, which reflects the degree of ketonuria, which, in turn, can be used to give a rough estimation of the level of hyperketonemia in the body (see table below). Alternatively, some products targeted to diabetics such as the Abbott Precision Xtra or the Nova Max can be used to take a blood sample and measure the β-hydroxybutyrate ketone levels directly. Normal serum reference ranges for ketone bodies are 0.5–3.0 mg/dL, equivalent to 0.05–0.29 mmol/L.
Also, when the body is in ketosis, one's breath may smell of acetone. This is due to the breakdown of acetoacetic acid into acetone and carbon dioxide which is exhaled through the lungs. Acetone is the chemical responsible for the smell of nail polish remover and some paint thinners.
Severity
The concentration of ketone bodies may vary depending on diet, exercise, degree of metabolic adaptation and genetic factors. Ketosis can be induced when a ketogenic diet is followed for more than 3 days. This induced ketosis is sometimes called nutritional ketosis. This table shows the concentrations typically seen under different conditions
Note that urine measurements may not reflect blood concentrations. Urine concentrations will be lower with greater hydration, and after adaptation to a ketogenic diet the amount lost in the urine may drop while the metabolism remains ketotic. Most urine strips only measure acetoacetate, while when ketosis is more severe the predominant ketone body is β-hydroxybutyrate. Unlike glucose, ketones are excreted into urine at any blood level. Ketoacidosis is a metabolic derangement that cannot occur in a healthy individual who can produce insulin, and should not be confused with physiologic ketosis.
Controversy
Some clinicians regard eliminating carbohydrates as unhealthy and dangerous. However, it is not necessary to eliminate carbohydrates from the diet completely in order to achieve a state of ketosis. Other clinicians regard ketosis as a safe biochemical process that occurs during the fat-burning state. Ketogenesis can occur solely from the byproduct of fat degradation: acetyl-CoA. Ketosis, which is accompanied by gluconeogenesis (the creation of glucose de novo from pyruvate), is the specific state with which some clinicians are concerned. However, it is unlikely for a normal functioning person to reach life-threatening levels of ketosis, defined as serum beta-hydroxybutyrate (B-OHB) levels above 15 millimolar (mM) compared to ketogenic diets among non diabetics which "rarely run serum B-OHB levels above 3 mM." This is avoided with proper basal secretion of pancreatic insulin. People who are unable to secrete basal insulin, such as type 1 diabetics and long-term type II diabetics, are liable to enter an unsafe level of ketosis, eventually resulting in a coma that requires emergency medical treatment. The anti-ketosis conclusions have been challenged by a number of doctors and advocates of low-carbohydrate diets, who dispute assertions that the body has a preference for glucose and that there are dangers associated with ketosis.
Inuit People
The Inuit are often cited as an example of a culture that has lived for hundreds of years on a low-carbohydrate diet. However, in multiple studies the traditional Inuit diet has not been shown to be a ketogenic diet. Not only have multiple researchers been unable to detect any evidence of ketosis resulting from the traditional Inuit diet, but the ratios of fatty-acid to glucose were observed to be well below the generally accepted level of ketogenesis. Furthermore, studies investigating the fat yields from fully dressed wild ungulates, and the dietary habits of the cultures who rely on them, suggest that they are too lean to support a ketogenic diet. With limited access to fat and carbohydrates, cultures such as the Nunamiut Eskimos—who relied heavily on caribou for subsistence—annually traded for fat and seaweed with coastal-dwelling Taremiut.
Some Inuit consume as much as 15-20% of their calories from carbohydrates, largely from the glycogen found in raw meats. Furthermore, the blubber, organs, muscle and skin of the diving marine mammals that the Inuit eat have significant glycogen stores that are able to delay postmortem degradation, particularly in cold weather.
Moreover, recent studies show that the Inuit have evolved a number of rare genetic adaptations that make them especially well suited to eat large amounts of omega-3 fat. And earlier studies showed that the Inuit have a very high frequency—68% to 81% in certain arctic coastal populations—of an extremely rare autosomal recessive mutation of the CPT1A gene—a key regulator of mitochondrial long-chain fatty-acid oxidation—which results in a rare metabolic disorder known as carnitine palmitoyltransferase 1A (CPT1A) deficiency and promotes hypoketotic hypoglycemia—low levels of ketones and low blood sugar. The condition presents symptoms of a fatty acid and ketogenesis disorder. However, it appears to be highly beneficial to the Inuit as it shunts free fatty acids away from liver cells to brown fat, for thermogenesis. Thus the mutation may help the Inuit stay warm by preferentially burning fatty acids for heat in brown fat cells. In addition to promoting low ketone levels, this disorder also typically results in hepatic encephalopathy (enlarged liver) and high infant mortality. Inuit have been observed to have enlarged livers with an increased capacity for gluconeogenesis, and have greater capacity for excreting urea to remove ammonia, a toxic byproduct of protein breakdown. Ethnographic texts have documented the Inuit's customary habit of snacking frequently and this may well be a direct consequence of their high prevalence of the CPT1A mutation as fasting, even for several hours, can be deleterious for individuals with that allele, particularly during strenuous exercise. The high frequency of the CPT1A mutation in the Inuit therefore suggests that it is an important adaptation to their low carbohydrate diet and their extreme environment.
The diet of the Inuit is perhaps oversimplified in order to simulate evidence supporting the viability of long term carbohydrate deprivation. In addition to the seaweed and glycogen carbohydrates mentioned above, the Inuit are able to access many plant sources as well. The stomach contents of caribou contain a large quantity of partially digested lichens and plants which were considered a delicacy. Reindeer moss and other lichens were also harvested directly. The extended daylight of the arctic summer led to a profusion of plant life, and plant parts including berries, roots and stems, as well as mushrooms were harvested. These could be preserved for use in winter, often by dipping in seal fat.
Adaptation
While it is believed that carbohydrate intake after exercise is the most effective way of replacing depleted glycogen stores, studies have shown that, after a period of 2–4 weeks of adaptation, physical endurance (as opposed to physical intensity) is unaffected by ketosis; as long as the diet contains high amounts of fat, relative to carbohydrates. Some clinicians refer to this period of keto-adaptation as the "Schwatka Imperative" after the explorer who first identified the transition period from glucose-adaptation to keto-adaptation.
Veterinary medicine
In dairy cattle, ketosis is a common ailment that usually occurs during the first weeks after giving birth to a calf. Ketosis is in these cases sometimes referred to as acetonemia. A study from 2011 revealed that whether ketosis is developed or not depends on the lipids a cow uses to create butterfat. Animals prone to ketosis mobilize fatty acids from adipose tissue, while robust animals create fatty acids from blood phosphatidylcholine (lecithin). Healthy animals can be recognized by high levels of milk glycerophosphocholine and low levels of milk phosphocholine. Point of care diagnostic tests are available and are reasonably useful.
In sheep, ketosis, evidenced by hyperketonemia with beta-hydroxybutyrate in blood over 0.7 mmol/L, occurs in pregnancy toxemia. This may develop in late pregnancy in ewes bearing multiple fetuses, and is associated with the considerable glucose demands of the conceptuses. In ruminants, because most glucose in the digestive tract is metabolized by rumen organisms, glucose must be supplied by gluconeogenesis, for which propionate (produced by rumen bacteria and absorbed across the rumen wall) is normally the principal substrate in sheep, with other gluconeogenic substrates increasing in importance when glucose demand is high or propionate is limited. Pregnancy toxemia is most likely to occur in late pregnancy because most fetal growth (and hence most glucose demand) occurs in the final weeks of gestation; it may be triggered by insufficient feed energy intake (anorexia due to weather conditions, stress or other causes), necessitating reliance on hydrolysis of stored triglyceride, with the glycerol moiety being used in gluconeogenesis and the fatty acid moieties being subject to oxidation, producing ketone bodies. Among ewes with pregnancy toxemia, beta-hydroxybutyrate in blood tends to be higher in those that die than in survivors. Prompt recovery may occur with natural parturition, Caesarean section or induced abortion. Prevention (through appropriate feeding and other management) is more effective than treatment of advanced stages of ovine ketosis.
