A ketene is an organic compound of the form R′R″C=C=O. Ketene also refers to CH2=C=O, occasionally called ethenone. Although they are highly useful, ketene and its various derivatives are rarely isolated.
Contents
Preparation
Ketenes were first studied as a class by Hermann Staudinger.
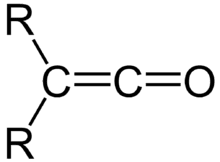
Ketene, the simplest ketene, is produced on a large scale industrially for use in the production of acetic anhydride. It can also be generated by pyrolysis (thermal cracking) of acetone:
CH3−CO−CH3 → CH2=C=O + CH4This reaction is called the Schmidlin ketene synthesis.
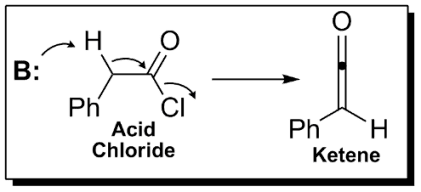
Ketenes can be prepared from acyl chlorides by an elimination reaction in which HCl is lost:
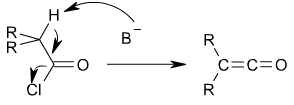
In this reaction, a base, usually triethylamine, removes the acidic proton alpha to the carbonyl group, inducing the formation of the carbon-carbon double bond and the loss of a chloride ion.
Ketenes can also be formed from α-diazoketones by Wolff rearrangement.
Another way to generate ketenes is through flash vacuum thermolysis with 2-pyridylamines. Plüg and Wentrup developed a method in 1997 that improved on FVT reactions to produce ketenes with a stable FVT that is moisture insensitive, using mild conditions (480 °C). The N-pyridylamines are prepared via a condensation with R-malonates with N-amino(pyridene) and DCC as the solvent.
Reactions and applications
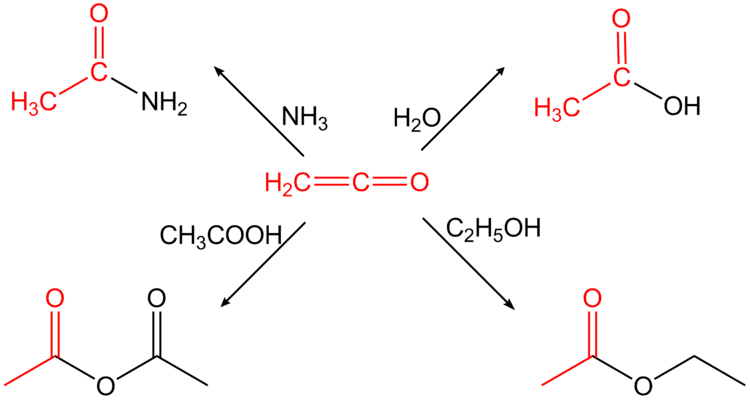
Ketenes are generally very reactive, and participate in various cycloadditions. One important process is the dimerization to give propiolactones. A specific examplethe dimerizaation of the ketene of stearic acid affords alkyl ketene dimers, which are widely used in the paper industry. AKD's react with the hydroxyl groups on the celluose via esterification reaction. The
They will also undergo [2+2] cycloaddition reactions with electron-rich alkynes to form cyclobutenones, or carbonyl groups to form beta-lactones. With imines beta-lactams are formed. This is the Staudinger synthesis, a facile route to this important class of compounds. With acetone, ketene reacts to give Isopropenyl acetate.
Reactions between diols (HO−R−OH) and bis-ketenes (O=C=CH−R′−CH=C=O) yield polyesters with a repeat unit of (−O−R−O−CO−R′−CO).
Ethyl acetoacetate, a important starting material in organic synthesis, can be prepared using a diketene in reaction with ethanol. They directly form ethyl acetoacetate, and the yield is high when carried out under controlled circumstances; this method is therefore used industrially.
