Formula HOF Boiling point 0 °C | Molar mass 36.0057 g/mol | |
 | ||
Related compounds Appearance pale yellow liquid above −117 °C; white solid below −117 °C | ||
Chemistry chemical bonding 12 of 35 lewis structures hypofluorous acid hof
Hypofluorous acid, HOF, is the only known oxoacid of fluorine. It is also the only hypohalous acid that can be isolated as a solid. HOF is an intermediate in the oxidation of water by fluorine, which produces hydrogen fluoride, oxygen difluoride, hydrogen peroxide, ozone and oxygen. HOF is explosive at room temperature, forming HF and O2:
Contents
It was isolated in the pure form by by passing F2 gas over ice at −40 °C, collecting the HOF gas, and condensing it:
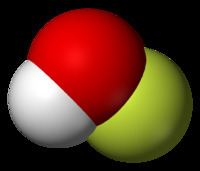
F2 + H2O → HOF + HFThe compound has been characterized in the solid phase by X-ray crystallography as a bent molecule with an angle of 101°. The O–F and O–H bond lengths are 144.2 and 96.4 picometres, respectively. The solid framework consists of chains with O–H···O linkages. The structure has also been analyzed in the gas phase, a state in which the H–O–F bond angle is slightly narrower (97.2°).
Hypofluorous acid in acetonitrile (generated in situ by passing gaseous fluorine through "wet" acetonitrile) serves as a highly electrophilic oxygen-transfer agent. Treating phenanthroline with this reagent yielded the previously elusive 1,10-phenanthroline dioxide, more than 50 years after the first unsuccessful attempt.
This molecule is not very symmetric since the atoms are all different. It only has two symmetry operations: identity (E) and a mirror plane that goes through the three atoms. The assigned point group is Cs.
Hypofluorites
Hypofluorites are derivatives of OF−, which is the conjugate base of hypofluorous acid. One example is trifluoromethyl hypofluorite (CF3OF).
