 | ||
Hydroperoxyl
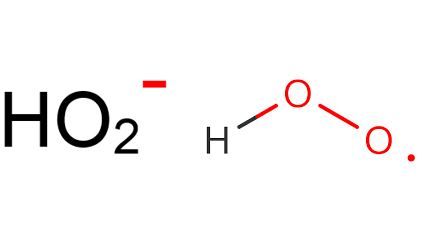
The hydroperoxyl radical, also known as the perhydroxyl radical, is the protonated form of superoxide with the chemical formula HO2.
Contents
What does hydroperoxyl mean
Formation
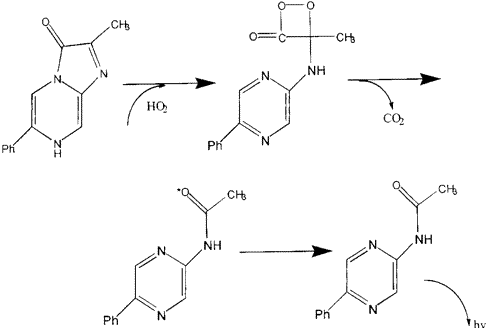
Hydroperoxyl is formed through the transfer of a hydrogen atom or even ion(s) to molecular oxygen, an oxygen atom to a hydroxyl radical or a proton to a superoxide anion. These radical type reactions are not fully understood.
Reference 1.The chemistry of carbonyl compounds in the atmosphere—a review P Carlier, H Hannachi, G Mouvier - Atmospheric Environment (1967), 1986 - Elsevier ... the dayt,me, carbonyl compound product,on ts therefore virtually controlled by the formation of free ra&cals from ... t to the double bond are by far the most reactive since the radical obtained ~s ... HO2 + Butane 4 0 x 10- ~6 Lloyd 1974 298 34 Reaction with O~ radicals Rate constant ... Cited by 453 Related articles All 4 versions Cite Save (Search machine-Google,08.01.2017)
Reactivity
The superoxide anion, O2−, and the hydroperoxyl radical are in equilibrium in aqueous solution:
O2− + H2O ⇌ HO2 + OH−The protonation/deprotonation equilibrium exhibits a pKa of 4.88; consequently, about 0.3% of any superoxide present in the cytosol of a typical cell is in the protonated form.
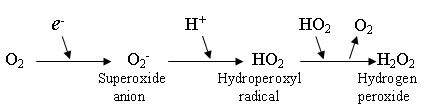
Unlike O2−, which predominantly acts as a reductant, HO2 can act as an oxidant in a number of biologically important reactions, such as the abstraction of hydrogen atoms from tocopherol and polyunstaturated fatty acids in the lipid bilayer. As such, it may be an important initiator of lipid peroxidation.
Because dielectric constant has a strong effect on pKa, and the dielectric constant of air is quite low, superoxide produced (photochemically) in the atmosphere is almost exclusively present as HO2. As HO2 is quite reactive, it acts as a "cleanser" of the atmosphere by degrading certain organic pollutants. As such, the chemistry of HO2 is of considerable geochemical importance.
Effect on environment

Gaseous hydroperoxyl is involved in reaction cycles that destroy atmospheric ozone. It can be formed as a result of the oxidation of hydrocarbons in the troposphere.
