Density 886 kg/m³ | ||
 | ||
Appearance Pale yellowish green clear liquid | ||
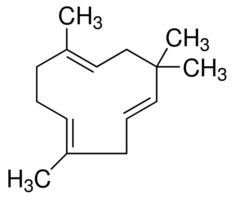
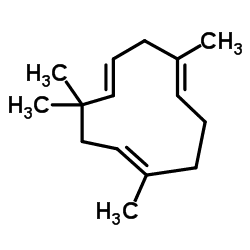
Humulene, also known as α-humulene or α-caryophyllene, is a naturally occurring monocyclic sesquiterpene (C15H24), containing an 11-membered ring and consisting of 3 isoprene units containing three nonconjugated C═C double bonds, two of them being triply substituted and one being doubly substituted. It was first found in the essential oils of Humulus lupulus (hops), from which it derives its name. Humulene is an isomer of β-caryophyllene, and the two are often found together as a mixture in many aromatic plants.
Contents
- Terpene description humulene
- Occurrence
- Preparation and synthesis
- Research
- Atmospheric chemistry
- References
Terpene description humulene
Occurrence

Humulene is one of the components of the essential oil from the flowering cone of the hops plant, Humulus lupulus, from which it derives its name. The concentration of humulene varies among different varieties of the plant but can be up to 40% of the essential oil. Humulene and its reaction products in the brewing process of beer gives many beers their “hoppy” aroma. Noble hop varieties have been found to have higher levels of humulene, while other bitter hop varieties contain low levels. Multiple epoxides of humulene are produced in the brewing process. In a scientific study involving gas chromatography–mass spectrometry analysis of samples and a trained sensory panel, it was found that the hydrolysis products of humulene epoxide II specifically produces a “hoppy” aroma in beer.
α-Humulene has been found in many aromatic plants on all continents, often together with its isomer β-caryophyllene. Proven α-humulene emitters into the atmosphere are pine trees, orange orchards, marsh elders, tobacco, and sunflower fields. α-Humulene is contained in the essential oils of aromatic plants such as Salvia officinalis (common sage, culinary sage), Lindera strychnifolia Uyaku or Japanese spicebush, ginseng species, up to 29.9% of the essential oils of Mentha spicata, the ginger family (Zingiberaceae), 10% of the leaf oil of Litsea mushaensis, a Chinese laurel tree, 4% of the leaf extract of Cordia verbenacea, a bush in coastal tropical South America (erva baleeira), but with 25% trans-Caryophyllene and is one of the chemical compounds that contribute to the taste of the spice Persicaria odorata or Vietnamese coriander and the characteristic aroma of Cannabis sativa.
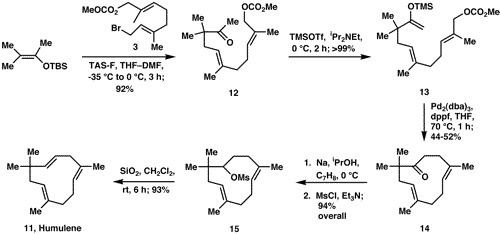
Preparation and synthesis
Humulene is one of many sesquiterpenoids that are derived from farnesyl diphosphate (FPP). The formation of humulene from FPP is catalyzed by sesquiterpene synthesis enzymes.
This biosynthesis can be mimicked in the laboratory by preparing allylic stanane from farnesol, termed Corey synthesis. There are diverse ways to synthesize humulene in the laboratory, involving differing closures of the C-C bond in the macrocycle. The McMurry synthesis uses a titanium-catalyzed carbonyl coupling reaction; the Takahashi synthesis uses intramolecular alkylation of an allyl halide by a protected cyanohydrin anion; the Suginome synthesis utilizes a geranyl fragment; and the de Groot synthesis synthesizes humulene from a crude distillate of eucalyptus oil. Humulene can also by synthesized using a combination of four-component assembly and palladium-mediated cyclization, outlined below. This synthesis is noteworthy for the simplicity of the C−C bond constructions and cyclization steps, which it is believed will prove advantageous in the synthesis of related polyterpenoids.
To understand humulene's regioselectivity,the fact that one of the two triply substituted C═C double bonds is significantly more reactive,its conformational space was explored computationally and four different conformations were identified.
Research
In laboratory studies, humulene is being studied for potential anti-inflammatory effects.
Atmospheric chemistry
α-Humulene is a biogenic volatile organic compound, emitted by numerous plants (see occurrence) with a relatively high potential for secondary organic aerosol formation in the atmosphere. It quickly reacts with ozone in sunlight (photooxidation) to form oxygenated products. α-Humulene has a very high reaction rate coefficient (1.17 × 10−14 cm3 molecule−1 s−1) compared to most monoterpenes. Since it contains three double bonds, first-, second- and third-generation products are possible that can each condense to form secondary organic aerosol. At typical tropospheric ozone mixing ratios of 30 ppb the lifetime of α-humulene is about 2 min, while the first- and second-generation products have average lifetimes of 1 h and 12.5 h, respectively.
