Species Human Entrez 6513 | Human Mouse Ensembl ENSG00000117394 | |
 | ||
Aliases SLC2A1, CSE, DYT17, DYT18, DYT9, EIG12, GLUT, GLUT-1, GLUT1, GLUT1DS, HTLVR, PED, SDCHCN, solute carrier family 2 member 1 External IDs OMIM: 138140 MGI: 95755 HomoloGene: 68520 GeneCards: SLC2A1 | ||
Glucose transporter 1 (or GLUT1), also known as solute carrier family 2, facilitated glucose transporter member 1 (SLC2A1), is a uniporter protein that in humans is encoded by the SLC2A1 gene. GLUT1 facilitates the transport of glucose across the plasma membranes of mammalian cells.
Contents
Discovery
GLUT1 was the first glucose transporter to be characterized. GLUT 1 is highly conserved. GLUT 1 of humans and mice have 98% identity at the amino acid level. GLUT 1 is encoded by the SLC2 gene and is one of a family of 14 genes encoding GLUT proteins.
Function
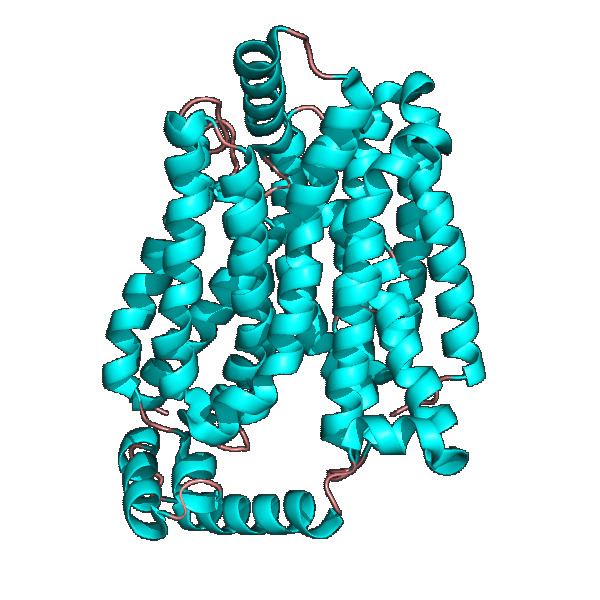
Energy-yielding metabolism in erythrocytes depends on a constant supply of glucose from the blood plasma, where the glucose concentration is maintained at about 5mM. Glucose enters the erythrocyte by facilitated diffusion via a specific glucose transporter, at a rate about 50,000 times greater than uncatalyzed transmembrane diffusion. The glucose transporter of erythrocytes (called GLUT1 to distinguish it from related glucose transporters in other tissues) is a type III integral protein with 12 hydrophobic segments, each of which is believed to form a membrane-spanning helix. The detailed structure of GLUT1 is not known yet, but one plausible model suggests that the side-by-side assembly of several helices produces a transmembrane channel lined with hydrophilic residues that can hydrogen-bond with glucose as it moves through the channel.
GLUT1 is responsible for the low level of basal glucose uptake required to sustain respiration in all cells. Expression levels of GLUT1 in cell membranes are increased by reduced glucose levels and decreased by increased glucose levels.
GLUT1 is also a major receptor for uptake of Vitamin C as well as glucose, especially in non vitamin C producing mammals as part of an adaptation to compensate by participating in a Vitamin C recycling process. In mammals that do produce Vitamin C, GLUT4 is often expressed instead of GLUT1.
Tissue distribution
It is widely distributed in fetal tissues. In the adult it is expressed at highest levels in erythrocytes and also in the endothelial cells of barrier tissues such as the blood–brain barrier.
Structure
GLUT1 behaves as a Michaelis-Menten enzyme and contains 12 membrane-spanning alpha helices, each containing 20 amino acid residues. A helical wheel analysis shows that the membrane spanning alpha helices are amphipathic, with one side being polar and the other side hydrophobic. Six of these membrane spanning helices are believed to bind together in the membrane to create a polar channel in the center through which glucose can traverse, with the hydrophobic regions on the outside of the channel adjacent to the fatty acid tails of the membrane.
Clinical significance
Mutations in the GLUT1 gene are responsible for GLUT1 deficiency or De Vivo disease, which is a rare autosomal dominant disorder. This disease is characterized by a low cerebrospinal fluid glucose concentration (hypoglycorrhachia), a type of neuroglycopenia, which results from impaired glucose transport across the blood–brain barrier.
GLUT1 is also a receptor used by the HTLV virus to gain entry into target cells.
Glut1 has also been demonstrated as a powerful histochemical marker for hemangioma of infancy
Interactions
GLUT1 has been shown to interact with GIPC1.
GLUT1 has two significant types in brain 45k and 55k. GLUT1 45k is present on astroglia of neurons and GLUT1 55k is present on capillaries in brain and is responsible for glucose transport across blood brain barrier and its deficiency causes low level of glucose in CSF (less than 60 mg/dl) which may manifest as convulsion in deficient individuals.
Recently it has been described a GLUT1 inhibitor, DERL3, that is often methylated in colorectal cancer. In this cancer, DERL3 methylations seems to mediate the Warburg Effect.
Inhibitors
Fasentin is a small molecule inhibitor of the intracellular domain of GLUT1 preventing glucose uptake.
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles.
