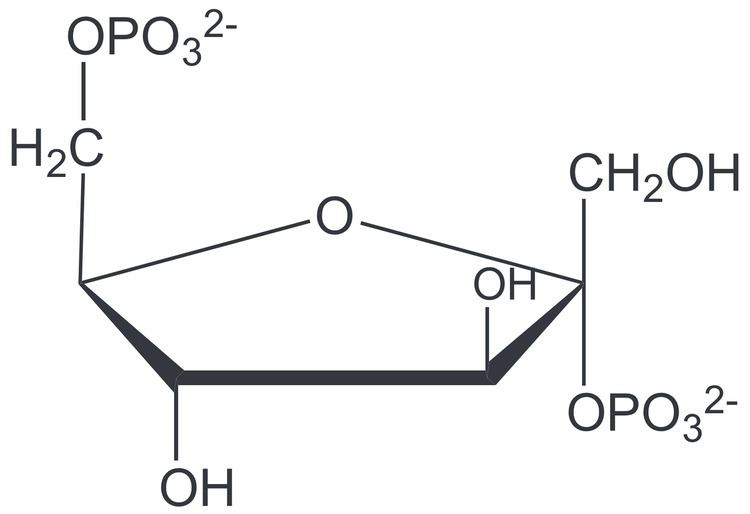
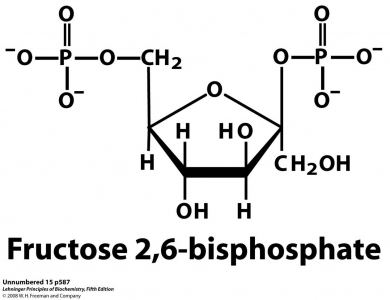
Formula C6H14O12P2 Boiling point 760.3 °C | Molar mass 340.116 g/mol | |
 | ||
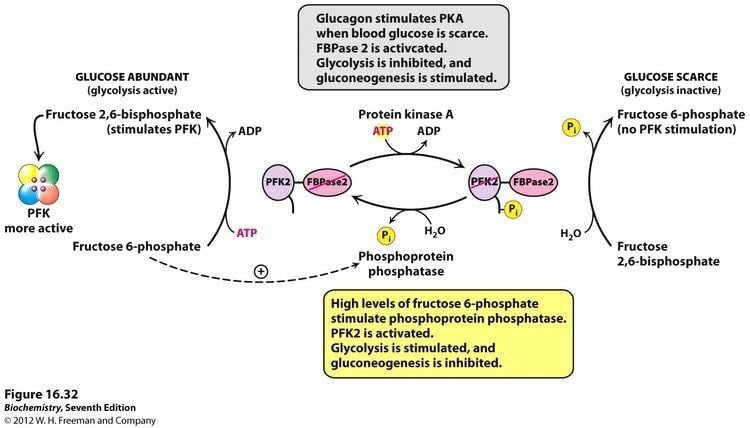
Fructose-2,6-bisphosphate abbreviated Fru-2,6-P2, is a metabolite that allosterically affects the activity of the enzymes phosphofructokinase 1 (PFK-1) and fructose 1,6-bisphosphatase (FBPase-1) to regulate glycolysis and gluconeogenesis. Fru-2,6-P2 is synthesized and broken down by the bifunctional enzyme phosphofructokinase 2/fructose-2,6-bisphosphatase (PFK-2/FBPase-2).
Contents
- Express Video of the Week Regulation by Fructose 26 biphosphate
- Effects on glucose metabolism
- Production regulation
- Regulation of sucrose production
- References
The synthesis of Fru-2,6-P2 is performed through a bifunctional enzyme containing both PFK-2 and FBPase-2, which is dephosphorylated, allowing the PFK-2 portion to phosphorylate fructose 6-phosphate using ATP. The breakdown of Fru-2,6-P2 is catalyzed by the phosphorylation of the bifunctional enzyme, which allows FBPase-2 to dephosphorylate Fructose-2,6-bisphosphate to produce Fructose 6-phosphate and Pi.
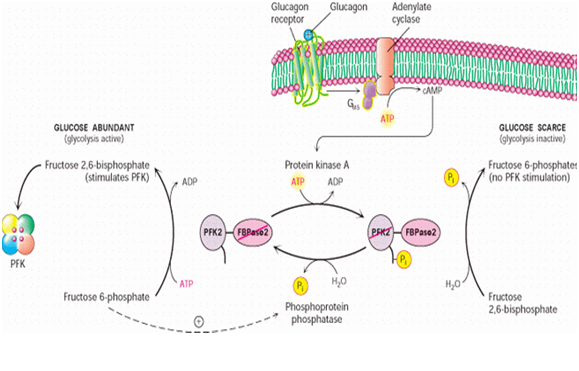
Reaction scheme of breakdown of fructose-2,6-bisphosphate to fructose-6-phosphate.
Express Video of the Week: Regulation by Fructose-2,6-biphosphate
Effects on glucose metabolism
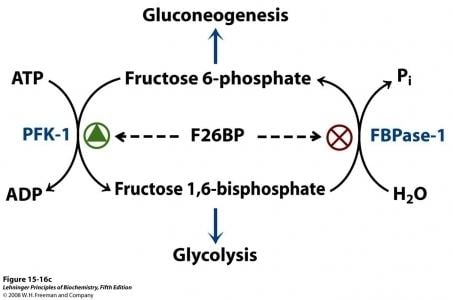
Fru-2,6-P2 strongly activates glucose breakdown in glycolysis through allosteric modulation of phosphofructokinase 1 (PFK-1). Elevated expression of Fru-2,6-P2 levels in the liver allosterically activates phosphofructokinase 1 by increasing the enzyme’s affinity for fructose 6-phosphate, while decreasing its affinity for inhibitory ATP and citrate. At physiological concentration, PFK-1 is almost completely inactive, but interaction with Fru-2,6-P2 activates the enzyme to stimulate glycolysis and enhance breakdown of glucose.
Production regulation
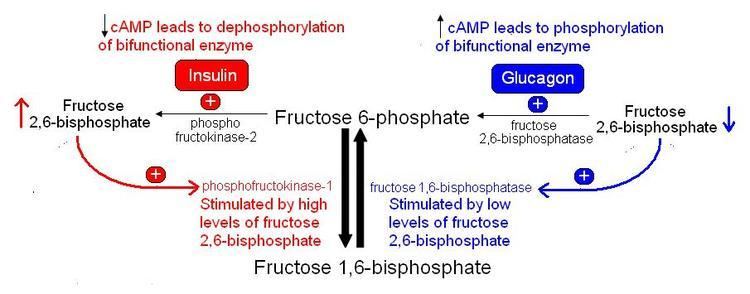
The concentration of Fru-2,6-P2 in cells is controlled through regulation of the synthesis and breakdown by PFK-2/FBPase-2. The primary regulators of this are the hormones insulin, glucagon, and epinephrine which affect the enzyme through phosphorlyation/dephosphorylation reactions. Release of the hormone glucagon triggers production of cyclic adenosine monophosphate (cAMP), which activates a cAMP-dependent protein kinase. This kinase phosphorylates the PFK-2/FBPase-2 enzyme at an NH2-terminal Ser residue with ATP to activate the FBPase-2 activity and inhibit the PFK-2 activity of the enzyme, thus reducing levels of Fru-2,6-P2 in the cell. With decreasing amounts of Fru-2,6-P2, glycolysis becomes inhibited while gluconeogenesis is activated. Insulin triggers the opposite response. As a phosphoprotein phosphatase, insulin dephosphorylates the enzyme, thus activating the PFK-2 and inhibiting the FBPase-2 activities. With additional Fru-2,6-P2 present, activation of PFK-1 occurs to stimulate glycolysis while inhibiting gluconeogenesis.
Regulation of sucrose production
Fru-2,6-P2 plays an important role in the regulation of triose phosphates, the end products of the Calvin Cycle. In the Calvin Cycle, 5/6th of triose phosphates are recycled to make ribulose 1,5-bisphosphate. The remaining 1/6 of triose phosphate can be converted into sucrose or stored as starch. Fru-2,6-P2 inhibits production of fructose 6-phosphate, a necessary element for sucrose synthesis. When the rate of photosynthesis in the light reactions is high, triose phosphates are constantly produced and the production of Fru-2,6-P2 is inhibited, thus producing sucrose. Fru-2,6-P2 production is activated when plants are in the dark and photosynthesis and triose phosphates are not produced.