Specialty endocrinology ICD-9-CM 277.31 DiseasesDB 9836 | ICD-10 E85.0 OMIM 249100 608107 MedlinePlus 000363 | |
 | ||
Familial Mediterranean fever (FMF) is a hereditary inflammatory disorder. FMF is an autoinflammatory disease caused by mutations in Mediterranean fever gene, which encodes a 781–amino acid protein called pyrin. While all ethnic groups are susceptible to FMF, it "usually occurs in people of Mediterranean origin—including Sephardic Jews, Mizrahi Jews, Armenians, Azerbaijanis, Arabs, Greeks, Turks and Italians".
Contents
The disorder has been given various names, including familial paroxysmal polyserositis, periodic peritonitis, recurrent polyserositis, benign paroxysmal peritonitis, periodic disease or periodic fever, Reimann periodic disease or Reimann's syndrome, Siegal-Cattan-Mamou disease, and Wolff periodic disease. Note that "periodic fever" can also refer to any of the periodic fever syndromes.
Attacks
There are seven types of attacks. Ninety percent of all patients have their first attack before they are 18 years old. All develop over 2–4 hours and last anywhere from 6 hours to 4 days. Most attacks involve fever.
Complications
AA-amyloidosis with kidney failure is a complication and may develop without overt crises. AA amyloid protein is produced in very large quantities during attacks, and at a low rate between them, and accumulates mainly in the kidney, as well as the heart, spleen, gastrointestinal tract, and thyroid.
There appears to be an increase in the risk for developing particular vasculitis-related diseases (e.g. Henoch–Schönlein purpura), spondylarthropathy, prolonged arthritis of certain joints and protracted myalgia.
Pathophysiology
Virtually all cases are due to a mutation in the Mediterranean Fever (MEFV) gene on the sixteenth chromosome, which codes for a protein called pyrin or marenostrin. Various mutations of this gene lead to FMF, although some mutations cause a more severe picture than others. Mutations occur mainly in exons 2, 3, 5 and 10.
The function of pyrin has not been completely elucidated, but it appears to be a suppressor of the activation of caspase 1, the enzyme that stimulates production of interleukin 1β, a cytokine central to the process of inflammation. In other words, an ineffective pyrin doesn't inhibit inflammation normally, resulting in inflammatory episodes of membranes at differing sites. It is not conclusively known what exactly sets off the attacks, and why overproduction of IL-1 would lead to particular symptoms in particular organs (e.g. joints or the peritoneal cavity).
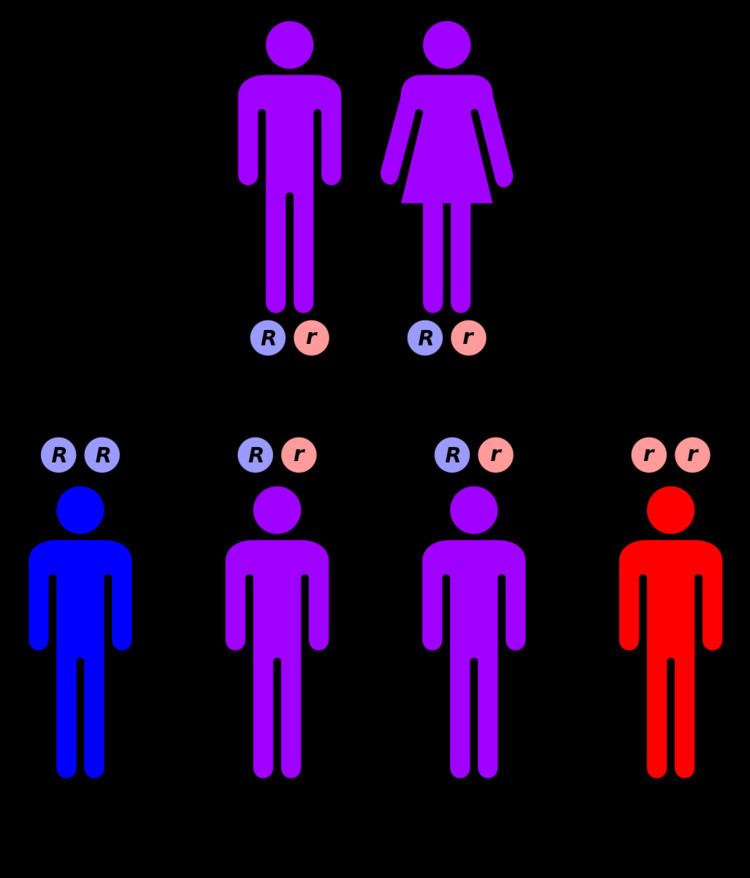
Genetics
The MEFV gene is located on the short arm of chromosome 16 (16p13). Many different mutations of the MEFV gene can cause the disorder. Having one mutation is unlikely to cause the condition. Having two mutations either a copy from both parents, or two different mutations, one from each parent is the threshold for a genetic diagnosis of FMF. However, most individuals who comply with the genetic diagnosis of FMF remain asymptomatic or undiagnosed. Whether this is due to modifier genes or environmental factors remains to be established.
Diagnosis
The diagnosis is clinically made on the basis of the history of typical attacks, especially in patients from the ethnic groups in which FMF is more highly prevalent. An acute phase response is present during attacks, with high C-reactive protein levels, an elevated white blood cell count and other markers of inflammation. In patients with a long history of attacks, monitoring the kidney function is of importance in predicting chronic kidney failure.
A genetic test is also available to detect mutations in the MEFV gene. Sequencing of exons 2, 3, 5, and 10 of this gene detects an estimated 97% of all known mutations.
A specific and highly sensitive test for FMF is the "Metaraminol Provocative Test (MPT)," whereby a single 10 mg infusion of Metaraminol is administered to the patient. A positive diagnosis is made if the patient presents with a typical, albeit milder, FMF attack within 48 hours. As MPT is more specific than sensitive, it does not identify all cases of FMF. Although a positive MPT can be very useful.
Treatment
Attacks are self-limiting, and require analgesia and NSAIDs (such as diclofenac). Colchicine, a drug otherwise mainly used in gout, decreases attack frequency in FMF patients. The exact way in which colchicine suppresses attacks is unclear. While this agent is not without side effects (such as abdominal pain and muscle pains), it may markedly improve quality of life in patients. The dosage is typically 1–2 mg a day. Development of amyloidosis is delayed with colchicine treatment. Interferon is being studied as a therapeutic modality. Some advise discontinuation of colchicine before and during pregnancy, but the data are inconsistent, and others feel it is safe to take colchicine during pregnancy.
Approximately 5–10% of FMF cases are resistant to colchicine therapy alone. In these cases, adding anakinra to the daily colchicine regimen has been successful.
Epidemiology
FMF affects groups of people originating from around the Mediterranean Sea (hence its name). It is prominently present in the Armenians, Sephardi Jews (and, to a much lesser extent, Ashkenazi Jews), Cypriots and Arabs.
History
A New York City allergist, Dr Sheppard Siegal, first described the attacks of peritonitis in 1945; he termed this "benign paroxysmal peritonitis", as the disease course was essentially benign. Dr Hobart Reimann, working in the American University in Beirut, described a more complete picture which he termed "periodic disease".
In 1972, colchicine was discovered to prevent attacks. The link to the MEFV gene was discovered in 1997 by two different groups, each working independently - the French FMF Consortium, and the International FMF Consortium.
