ATC code none PubChem CID 5359421 | CAS Number 509-60-4 Molar mass 287.354 g/mol | |
 | ||
Routes ofadministration Oral, Intravenous, Intranasally, Sublingually Legal status AU: S8 (Controlled)CA: Schedule IDE: Anlage II (Prohibited)US: Schedule I Synonyms Dihydromorphine, Paramorphan | ||
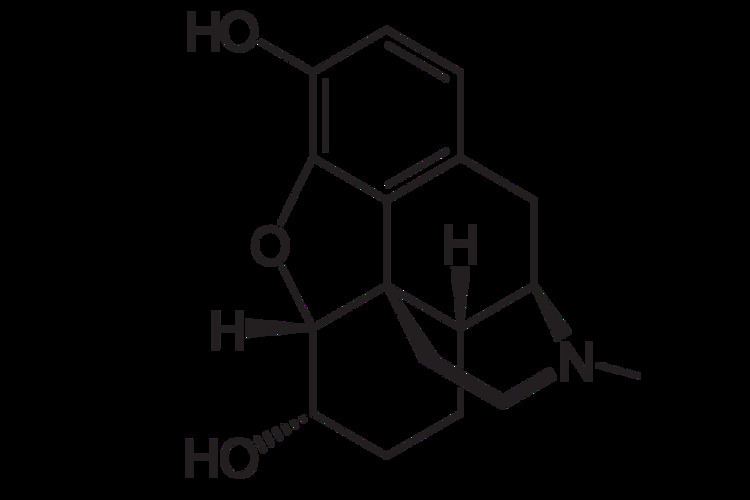
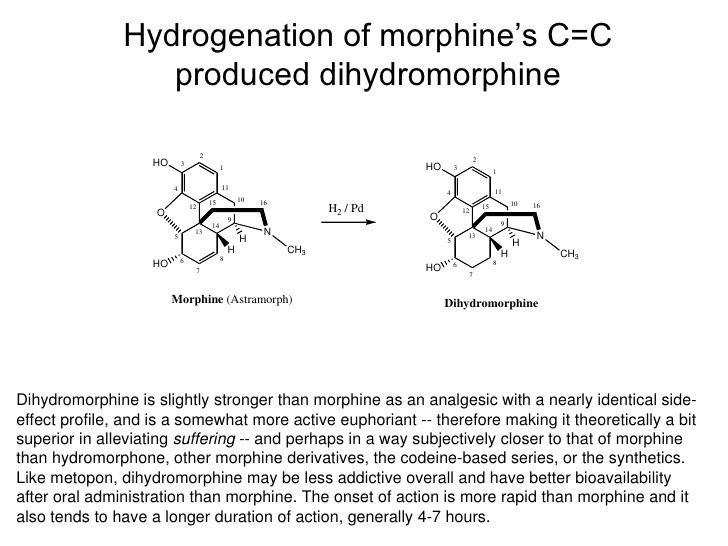
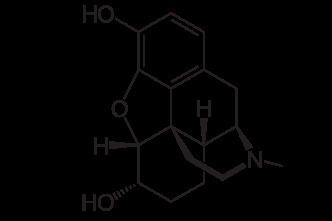
Dihydromorphine (Paramorfan, Paramorphan) is a semi-synthetic opioid structurally related to and derived from morphine. The 7,8-double bond in morphine is reduced to a single bond to get dihydromorphine. Dihydromorphine is a moderately strong analgesic and is used clinically in the treatment of pain and also is an active metabolite of the analgesic opioid drug dihydrocodeine. Dihydromorphine occurs in trace quantities in assays of opium on occasion, as does dihydrocodeine, dihydrothebaine, tetrahydrothebaine, etc. The process for manufacturing dihydromorphine from morphine for pharmaceutical use was developed in Germany in the late 19th Century, with the synthesis being published in 1900 and the drug introduced clinically as Paramorfan shortly thereafter. A high-yield synthesis from tetrahydrothebaine was later developed.
Contents
- Medical
- Research
- Strength
- Pharmacology
- Pharmacokinetics
- Legality
- United States of America
- Europe
- Japan
- References
Medical
Dihydromorphine is an opioid that is used for the management of moderate to severe pain such as cancer, although it's less effective in treating things such as neuropathic pain and is generally considered inappropriate and ineffective for psychological pain.
Research
Dihydromorphine, often labelled with the isotope tritium in the form of [3H]-dihydromorphine, is used in scientific research to study binding of the opioid receptors in the nervous system.
Strength

Dihydromorphine is slightly stronger than morphine as an analgesic with a similar side effect profile. The relative potency of dihydromorphine is about 1.2 times that of morphine. In comparison, the relative potency of dihydrocodeine is 1.15 times that of codeine.
Pharmacology
Dihydromorphine acts as an agonist at the μ-opioid (mu), δ-opioid (delta) and κ-opioid (kappa) receptors. Agonist of the μ-opioid and δ-opioid receptors is largely responsible for the clinical effects of opioids like dihydromorphine with the μ agonism providing more analgesia than the δ.
Pharmacokinetics

Dihydromorphine's onset of action is more rapid than morphine and it also tends to have a longer duration of action, generally 4–7 hours.
Legality
Under the 1961 international Single Convention on Narcotic Drugs treaty dihydromorphine is a Schedule I narcotic subject to control, and other countries' laws may vary.
United States of America
Under the Controlled Substances Act, dihydromorphine is listed as a Schedule I substance along with heroin. In the United States, its role in the production of dihydrocodeine and other related drugs make it a Schedule I substance with one of the higher annual manufacturing quotas granted by the US Drug Enforcement Administration—3300 kilos in 2013; manufacturers, distributors, and importers with the correct DEA license and state permits related thereto are able to use Schedule I drugs in this fashion when they are transformed into something of a lower schedule. The DEA has assigned dihydromorphine and all of its salts, esters, &c the ACSCN of 9145. As with nicomorphine, MDMA, heroin (DEA 2013 production quota: 25 grammes) and the like, dihydromorphine is also used in research such as that mentioned above in properly licensed facilities; Form 225, the most common and least expensive individual researcher's license, does not include Schedule I so the lab must have a higher-level DEA registration As with other licit opioids used for medical purposes in other countries, including even much weaker opioids like nicocodeine, benzylmorphine, and tilidine, the reason for dihydromorphine being in Schedule I is that it was not in medical use in the USA at time the Controlled Substances Act of 1970 was drawn up.
Europe
Dihydromorphine is regulated in the same fashion as morphine in Germany under the BtMG, Austrian SMG, and Swiss BtMG, where it is still used as an analgesic. The drug was invented in Germany in 1900 and marketed shortly thereafter. It is often used in Patient Controlled Analgesia units.
Japan
Dihydromorphine and morphine are also used alongside each other in clinical use in Japan and is regulated as such
