ICD-10 C83.3 eMedicine article/202969 | ICD-O M9680/3 MeSH D016403 | |
 | ||
Specialty Hematology and oncology | ||
Diffuse large B-cell lymphoma (DLBCL or DLBL) is a cancer of B cells, a type of white blood cell responsible for producing antibodies. It is the most common type of non-Hodgkin lymphoma among adults, with an annual incidence of 7–8 cases per 100,000 people per year. This cancer occurs primarily in older individuals, with a median age of diagnosis at approximately 70 years of age, though it can also occur in children and young adults in rare cases. DLBCL is an aggressive tumor which can arise in virtually any part of the body, and the first sign of this illness is typically the observation of a rapidly growing mass, sometimes associated with B symptoms—fever, weight loss, and night sweats.
Contents
- Classification
- Morphology
- Gene and microRNA expression
- Immunohistochemistry
- Signs and symptoms
- Chemotherapy
- Radiation therapy
- Prognosis
- Research
- Recent studies
- Stage 1
- Stage 2
- Stage 3
- Results
- References
The causes of diffuse large B-cell lymphoma are not well understood. Usually DLBCL arises from normal B cells, but it can also represent a malignant transformation of other types of lymphoma or leukemia. An underlying immunodeficiency is a significant risk factor. Infection with Epstein–Barr virus has also been found to contribute to the development of some subgroups of DLBCL.
Diagnosis of DLBCL is made by removing a portion of the tumor through a biopsy, and then examining this tissue using a microscope. Usually a hematopathologist makes this diagnosis. Several subtypes of DLBCL have been identified, each having a different clinical presentation and prognosis. However, the usual treatment for each of these is chemotherapy, often in combination with an antibody targeted at the tumor cells. Through these treatments, more than half of patients with DLBCL can be cured, and the overall five-year survival rate for older adults is around 58%.
Classification
Diffuse large B-cell lymphoma encompasses a biologically and clinically diverse set of diseases, many of which cannot be separated from one another by well-defined and widely accepted criteria. The World Health Organization (WHO) classification system defines more than a dozen subtypes, each of which can be differentiated based on the location of the tumor, the presence of other cells within the tumor (such as T cells), and whether the patient has certain other illnesses related to DLBCL. One of these well-defined groupings of particular note is "primary mediastinal (thymic) large B cell lymphoma", which arises within the thymus or mediastinal lymph nodes.
In some cases, a tumor may share many features with both DLBCL and Burkitt's lymphoma. In these situations, the tumor is classified as simply “B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma”. A similar situation can arise between DLBCL and Hodgkin's lymphoma; the tumor is then classified as “B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Hodgkin’s lymphoma”.
When a case of DLBCL does not conform to any of these subtypes, and is also not considered unclassifiable, then it is classified as “diffuse large B-cell lymphoma, not otherwise specified” (DLBCL, NOS). The majority of DLBCL cases fall into this category. Much research has been devoted to separating this still-heterogeneous group; such distinctions are usually made along lines of cellular morphology, gene expression, and immunohistochemical properties.
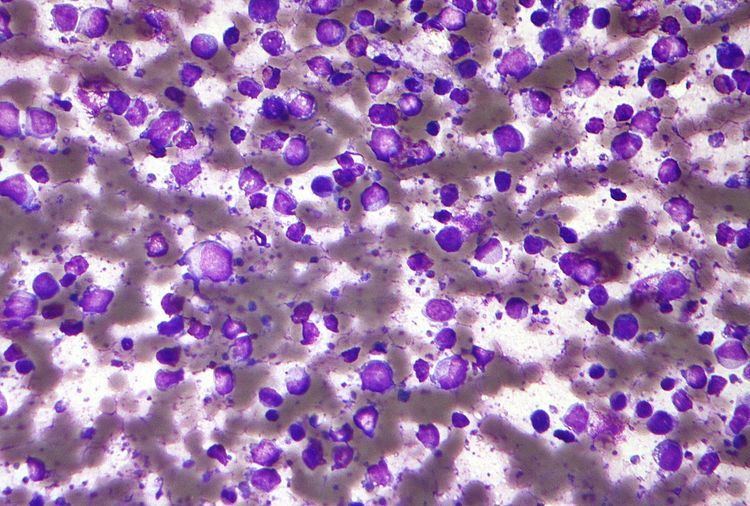
Morphology
Within cellular morphology, three variants are most commonly seen: centroblastic, immunoblastic, and anaplastic.
Most cases of DLBCL are centroblastic, having the appearance of medium-to-large-sized lymphocytes with scanty cytoplasm. Oval or round nuclei containing fine chromatin are prominently visible, having two to four nucleoli within each nucleus. Sometimes the tumor may be monomorphic, composed almost entirely of centroblasts. However, most cases are polymorphic, with a mixture of centroblastic and immunoblastic cells.
Immunoblasts have significant basophilic cytoplasm and a central nucleolus. A tumor can be classified as immunoblastic if greater than 90% of its cells are immunoblasts.This distinction can be problematic, however, because hematopathologists reviewing the microscope slides may often disagree on whether a collection of cells is best characterized as centroblasts or immunoblasts. Such disagreement indicates poor inter-rater reliability.
The third morphologic variant, anaplastic, consists of tumor cells which appear very differently from their normal B cell counterparts. The cells are generally very large with a round, oval, or polygonal shape and pleomorphic nuclei, and may resemble Reed-Sternberg cells.
Gene and microRNA expression
Gene expression profiling studies have also attempted to distinguish heterogeneous groups of DLBCL from each other. These studies examine thousands of genes simultaneously using a DNA microarray, looking for patterns which may help in grouping cases of DLBCL. Many studies now suggest that cases of DLBCL, NOS can be separated into two groups on the basis of their gene expression profiles; these groups are known as germinal center B-cell-like (GCB) and activated B-cell-like (ABC). Tumor cells in the germinal center B-cell-like subgroup resemble normal B cells in the germinal center closely, and are generally associated with a favorable prognosis. Activated B-cell-like tumor cells are associated with a poorer prognosis, and derive their name from studies which show the continuous activation of certain pathways normally activated when B cells interact with an antigen. The NF-κB pathway, which is normally involved in transforming B cells into plasma cells, is an important example of one such pathway.
Another notable finding of recent gene expression studies is the importance of the cells and microscopic structures interspersed between the malignant B cells within the DLBCL tumor, an area commonly known as the tumor microenvironment. The presence of gene expression signatures commonly associated with macrophages, T cells, and remodelling of the extracellular matrix seems to be associated with an improved prognosis and better overall survival. Alternatively, expression of genes coding for pro-angiogenic factors is correlated with poorer survival.
Recently, it was described that short non-coding RNAs named microRNAs (miRNAs) have important functions in lymphoma biology. In malignant B cells miRNAs participate in pathways fundamental to B cell development like B cell receptor (BCR) signalling, B cell migration/adhesion, cell-cell interactions in immune niches, and the production and class-switching of immunoglobulins. MiRNAs influence B cell maturation, generation of pre-, marginal zone, follicular, B1, plasma and memory B cells.
Immunohistochemistry
With the apparent success of gene expression profiling in separating biologically distinct cases of DLBCL, NOS, some researchers examined whether a similar distinction could be made using immunohistochemical staining (IHC), a widely used method for characterizing tissue samples. This technique uses highly specific antibody-based stains to detect proteins on a microscope slide, and since microarrays are not widely available for routine clinical use, IHC is a desirable alternative. Many of these studies focused on stains against the products of prognostically significant genes which had been implicated in DLBCL gene expression studies. Examples of such genes include BCL2, BCL6, MUM1, LMO2, MYC, and p21. Several algorithms for separating DLBCL cases by IHC arose out of this research, categorizing tissue samples into groups most commonly known as GCB and non-GCB. The correlation between these GCB/non-GCB immunohistochemical groupings and the GCB/ABC groupings used in gene expression profiling studies is uncertain, as is their prognostic value. This uncertainty may arise in part due to poor inter-rater reliability in performing common immunohistochemical stains.
Signs and symptoms
The most typical symptom at the time of diagnosis is a mass that is rapidly enlarging and located in a part of the body with multiple lymph nodes.
Chemotherapy
Current treatment typically includes R-CHOP, which consists of the traditional CHOP, to which rituximab has been added. This regimen has increased the rate of complete response for DLBCL patients, particularly in elderly patients.R-CHOP is a combination of one monoclonal antibody (rituximab), three chemotherapy agents (cyclophosphamide, doxorubicin, vincristine), and one steroid (prednisone). These drugs are administered intravenously, and the regimen is most effective when it is administered multiple times over a period of months. People often receive this type of chemotherapy through a PICC line (peripherally inserted central catheter) in their arm near the elbow or a surgically implanted venous access port. The number of cycles of chemotherapy given depends on the stage of the disease — patients with limited disease typically receive three cycles of chemotherapy, while patients with extensive disease may need to undergo six to eight cycles. A recent approach involves obtaining a PET scan after the completion of two cycles of chemotherapy, to assist the treatment team in making further decisions about the future course of treatment.Older people often have more difficulty tolerating therapy than younger people. Lower intensity regimens have been attempted in this age group.
Radiation therapy
Radiation therapy is often part of the treatment for DLBCL. It is commonly used after the completion of chemotherapy. Radiation therapy alone is not an effective treatment for this disease.
Prognosis
The germinal center subtype has the best prognosis,with 66.6% of treated patients surviving more than five years. The IPI score is used in prognosis in clinical practice. Lenalidomide has been recently shown to improve outcomes in the non-germinal center subtype. Ratios of immune effectors such as CD4 and CD8 to immune checkpoints such as PD-L1 and M2 macrophages are independent of and additive to the cell of origin and IPI in DLBCL, and are applicable to paraffin-embedded biopsy specimens. These findings might have potential implications for selection of patients for checkpoint blockade and/or lenalidomide within clinical trials.
For children with diffuse large B-cell lymphomas, most studies have found 5-year survival rates ranging from about 70% to more than 90%.
Research
A second regimen under evaluation is R-EPOCH (rituximab with etoposide-prednisone-vincristine-doxorubicin-cyclophosphamide), which demonstrated a 5-year progression-free survival (PFS) of 79% in a phase II trial. A phase III trial, CALGB 50303, is now comparing R-EPOCH with R-CHOP in patients with newly diagnosed DLBCL.
One area of active research is on separating patients into groups based on their prognosis and how likely they are to benefit from different drugs. Methods like gene expression profiling and next-generation sequencing may result in more effective and more personalized treatment.
Recent studies
James Cerhan and colleagues, try to determine genetic susceptibility that exists for this cancer by meta-analysis of three genome-wide association studies (GWAS). For this, a total of 3,857 cases and 7,666 controls were analyzed. This study is divided into three stages, which can differentiate into two phases:
– Discovery Phase: Stages 1 and 2.
– Phase replication: Stage 3.
Stage 1
At this early stage, to study the genetic susceptibility, a GWAS with DLBCL cases and controls of European ancestry from 22 studies of non-Hodgkin lymphoma (NHL) was performed. To determine the subtype of NHL, hierarchical classification proposed by the World Health Organization (WHO) was used. All cases of DLBCL with enough DNA and a subset of controls, matched for age and sex, along with 4% duplicates were genotyped. They were selected in this stage 611.844 single-nucleotide polymorphisms (SNP) that exceeded the quality criteria, genomic significance values, alignment and other statistical values.
Stage 2
At this stage the data of three independent previous GWAS, including two unpublished so far (GELA/EPIC and May) and one already published (USCF), with a total of 1,196 cases and 1,445 controls. The analysis was restricted to common SNPs on the basis of the 1000 Genomes Project version 3 because the data used were from different platforms. The criteria of quality control for these studies were adjusted to analyze all cases under the same conditions. In the meta-analysis of all SNPs of steps 1 and 2, 19 significant SNPs were identified, and 134 with a suggestive level of significance; 123 of the total were located in the HLA region on chromosome 6.
Stage 3
In the last stage, replication studies and technical validation were performed. The genotyping of 8 SNPs de novo was performed in the most significant HLA loci outside the region and one within it.
Results
As a result of this study, five SNPs were obtained in four loci significantly associated with the disease, which may be related to the following genes: EXOC2, PVT1, NCOA1 and HLA-B.
