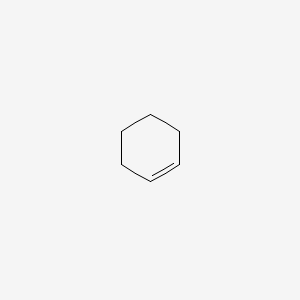
Formula C6H10 Density 811 kg/m³ | Molar mass 82.143 g/mol | |
 | ||
Cyclohexene chair
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes. Cyclohexene is not very stable upon long term storage with exposure to light and air because it forms peroxides.
Contents

Production and uses

Cyclohexene is produced by the partial hydrogenation of benzene, a process developed by the Asahi Chemical company. It is converted to cyclohexanol, which is dehydrogenated to give cyclohexanone, a precursor to caprolactam. Cyclohexene is also a precursor to adipic acid, maleic acid, dicyclohexyladipate, and cyclohexene oxide. Furthermore, it is used as a solvent.
Synthesis

A common experiment for beginning organic chemistry students is the acid-catalyzed dehydration of cyclohexanol with distillative removal of the resulting cyclohexene from the reaction mixture:
Oxidative cleavage

A green chemistry experiment is the oxidative cleavage of cyclohexene to form adipic acid. Hydrogen peroxide is used as the oxidant, in the presence of a tungsten catalyst.
Structure

Cyclohexene is most stable in a half-chair conformation, unlike the preference for a chair for of cyclohexane. One basis for the cyclohexane conformational preference for a chair is that it allows each bond of the ring to adopt a staggered conformation. For cyclohexene, however, the alkene is planar, equivalent to an eclipsed conformation at that bond.