Related compounds Appearance red liquid | Density 1.27 g/cm³ | |
 | ||
Carbon subsulfide is an inorganic chemical compound with the formula C3S2. This deep red liquid is immiscible with water but soluble in organic solvents. It readily polymerizes at room temperature to form a hard black solid.
Contents
Synthesis and structure
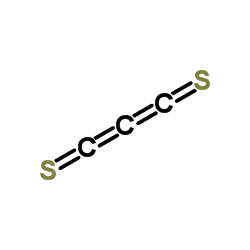
C3S2 was discovered by Béla Lengyel, who assigned it an unsymmetrical structure. Later, infrared and Raman spectroscopy showed that the structure is symmetrical with a D∞h point group symmetry, i.e. S=C=C=C=S. This compound is analogous to carbon suboxide whose structure is O=C=C=C=O.
Lengyel first synthesized this compound by passing carbon disulfide (CS2) vapor through an electric arc with carbon electrodes. This treatment produced a black solution that after filtration and evaporation gave a cherry-red liquid. He determined the molecular mass by cryoscopy. Later preparations of C3S2 include thermolysis of a stream of CS2 in a quartz tube heated to 900 to 1100 °C as well as flash vacuum pyrolysis (FVP) of 1,2-dithiole-3-thiones.
Reactions and occurrence
Among its few known reactions, C3S2 reacts with bromine to form the cyclic disulfide.
C3S2 polymerizes under applied pressure to give a black semi-conducting solid. A similar pressure-induced polymerization of CS2 also gives a black semiconducting polymer.
In addition, reactions of C3S2 can yield highly condensed sulfur-containing compounds, e.g. the reaction of C3S2 with 2-aminopyridine.
Using microwave spectroscopy, small CnS2 clusters have been detected in interstellar medium. The rotational transitions of these molecular carbon sulfides matched with the corresponding molecules.
