 | ||
Carbometalation is an organometallic reaction involving the nucleophilic addition to alkenes and alkynes of a diverse range of organometallic reagents such as organolithium compounds, organocopper compounds and Grignard reagents according to the following general alkyne scheme:
Contents
The addition can yield the cis or trans isomer and with unsymmetrical alkynes the organometallic compound can add in two different way thus control of regioselectivity is important.
In a follow-up step the sensitive metalalkenyl group is replaced by an electrophile E+.
Scope
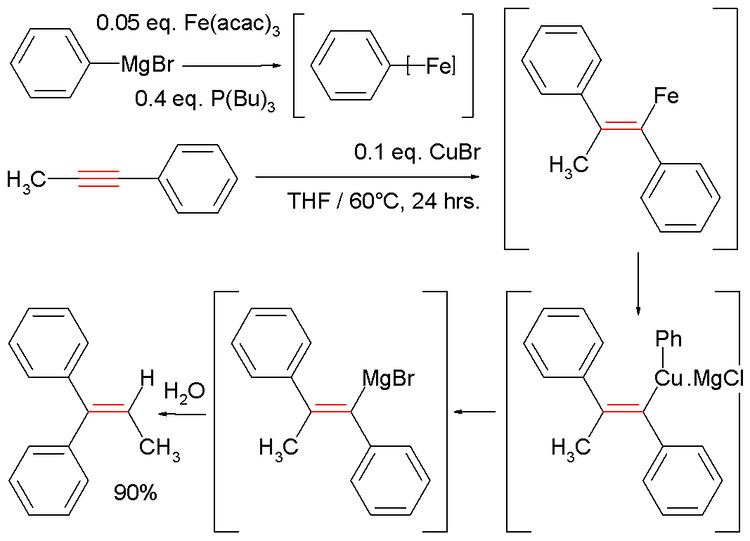
In one study methylphenylacetylene is reacted with phenylmagnesium bromide to a vinyl magnesium bromide which is quenched with water:
Another demonstration of this reaction type is an alternative route to tamoxifen starting from diphenylacetylene and ethyllithium:
The capturing electrophile here is triisopropyl borate forming the boronic acid R–B(OH)2. The second step completing tamoxifen is a Suzuki reaction.
Silylmetalation
This reaction type can be extended to compounds of silicon bonded to a suitable metal in so-called silylmetalation as an extension to hydrosilylation. In one study a zinc ate complex is formed from dimethylphenylsilyllithium, 2,2′-biphenol, zinc chloride and the Grignard reagent of tert-butylchloride which is capable of silylmetalation to styrene with 100% regioselectivity:
The intermediate complex can be captured with many electrophiles such as propargyl bromide (pictured, forming the allene), benzoyl chloride and allyl bromide.
