Formula CdS | Density 4.82 g/cm³ | |
 | ||
Appearance Yellow-orange to brown solid. | ||
Cadmium chemistry cadmium sulfide via sodium polysulfide
Cadmium sulfide is the inorganic compound with the formula CdS. Cadmium sulfide is a yellow solid. It occurs in nature with two different crystal structures as the rare minerals greenockite and hawleyite, but is more prevalent as an impurity substituent in the similarly structured zinc ores sphalerite and wurtzite, which are the major economic sources of cadmium. As a compound that is easy to isolate and purify, it is the principal source of cadmium for all commercial applications. Its vivid yellow color led to its adoption as a pigment for the yellow paint "cadmium yellow" in the 18th century.
Contents
- Cadmium chemistry cadmium sulfide via sodium polysulfide
- Production
- Routes to thin films of CdS
- Reactions
- Structure and physical properties
- Pigment
- Historical use in art
- CdS CdSe solutions
- Safety
- References

Production

Cadmium sulfide can be prepared by the precipitation from soluble cadmium(II) salts with sulfide ion. This reaction has been used for gravimetric analysis and qualitative inorganic analysis.
The preparative route and the subsequent treatment of the product, affects the polymorphic form that is produced (i.e., cubic vs hexagonal). It has been asserted that chemical precipitation methods result in the cubic zincblende form.

Pigment production usually involves the precipitation of CdS, the washing of the solid precipitate to remove soluble cadmium salts followed by calcination (roasting) to convert it to the hexagonal form followed by milling to produce a powder. When cadmium sulfide selenides are required the CdSe is co-precipitated with CdS and the cadmium sulfoselenide is created during the calcination step.
Cadmium sulfide is sometimes associated with sulfate reducing bacteria.
Routes to thin films of CdS
Special methods are used to produce films of CdS as components in some photoresistors and solar cells. In the chemical bath deposition method, thin films of CdS have been prepared using thiourea as the source of sulfide anions and an ammonium buffer solution to control pH:
Cd2+ + H2O + (NH2)2CS + 2 NH3 → CdS + (NH2)2CO + 2 NH4+
Cadmium sulfide can be produced using metalorganic vapour phase epitaxy and MOCVD techniques. This process requies volatile cadmium and sulfur precursors. A common example is the reaction of dimethylcadmium with diethyl sulfide:
Cd(CH3)2 + Et2S → CdS + CH3CH3 + C4H10Many other methods have been reported.
Other methods to produce films of CdS include
Reactions
Cadmium sulfide is soluble in (actually degraded by) acids, and this conversion has been investigated as a method of extracting the pigment from waste polymers e.g. HDPE pipes:
CdS + 2 HCl → CdCl2 + H2SWhen solutions of sulfide solutions containing dispersed CdS particles are irradiated with light hydrogen gas is generated:
H2S → H2 + S ΔHf = +9.4 kcal/molThe proposed mechanism involves the electron/hole pairs created when incident light is absorbed by the cadmium sulfide followed by these reacting with water and sulfide:
Production of an electron hole pairReaction of electronReaction of holeStructure and physical properties
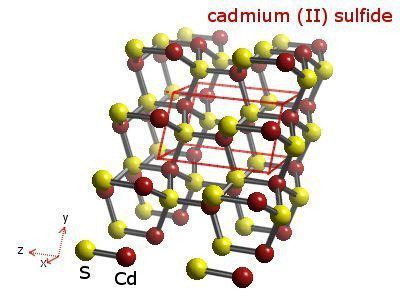
Cadmium sulfide has, like zinc sulfide, two crystal forms. The more stable hexagonal wurtzite structure (found in the mineral Greenockite) and the cubic zinc blende structure (found in the mineral Hawleyite). In both of these forms the cadmium and sulfur atoms are four coordinate. There is also a high pressure form with the NaCl rock salt structure.
Cadmium sulfide is a direct band gap semiconductor (gap 2.42 eV). The magnitude of its band gap means that it appears coloured.
As well as this obvious property other properties result:
Thin films of Cadmium Sulfide can be piezoelectric and have been used as transducers which can operate at frequencies in the GHz region.
Pigment
Synthetic cadmium pigments based on cadmium sulfide are valued for their good thermal stability, light and weather fastness, chemical resistance and high opacity. As a pigment, CdS is known as cadmium yellow. (CI pigment yellow 37) About 2000 tons are produced annually as of 1982, representing about 25% of the cadmium processed commercially. CdS is used as pigment in plastics.
Historical use in art
The general commercial availability of cadmium sulfide from the 1840s led to its adoption by artists, notably Van Gogh, Monet (in his London series and other works) and Matisse (Bathers by a river 1916–1919). The presence of cadmium in paints has been used to detect forgeries in paintings alleged to have been produced prior to the 19th century.
CdS-CdSe solutions
CdS and CdSe form solid solutions. Increasing amounts of cadmium selenide, gives pigments verging toward red, for example CI pigment orange 20 and CI pigment red 108.
Such solid solutions are components of photoresistors (light dependent resistors) sensitive to visible and near infrared light.
Safety
Cadmium sulfide is toxic, especially when inhaled as dust, and cadmium compounds general are classified as carcinogenic. Problems of biocompatibility have been reported when CdS is used as colors in tattoos.
