Symbol CRAL_TRIO InterPro IPR001251 SCOP 1aua | Pfam PF00650 SUPERFAMILY 1aua | |
 | ||
CRAL-TRIO domain is a protein structural domain that binds small lipophilic molecules. This domain is named after cellular retinaldehyde-binding protein (CRALBP) and TRIO guanine exchange factor.
Contents
CRALB protein carries 11-cis-retinol or 11-cis-retinaldehyde. It modulates interaction of retinoids with visual cycle enzymes. TRIO is involved in coordinating actin remodeling, which is necessary for cell migration and growth.
Other members of the family are alpha-tocopherol transfer protein and phosphatidylinositol-transfer protein (Sec14). They transport their substrates (alpha-tocopherol and phosphatidylinositol or phosphatidylcholine, respectively) between different intracellular membranes. Family also include a guanine nucleotide exchange factor that may function as an effector of RAC1 small G-protein.
The N-terminal domain of yeast ECM25 protein has been identified as containing a lipid binding CRAL-TRIO domain.
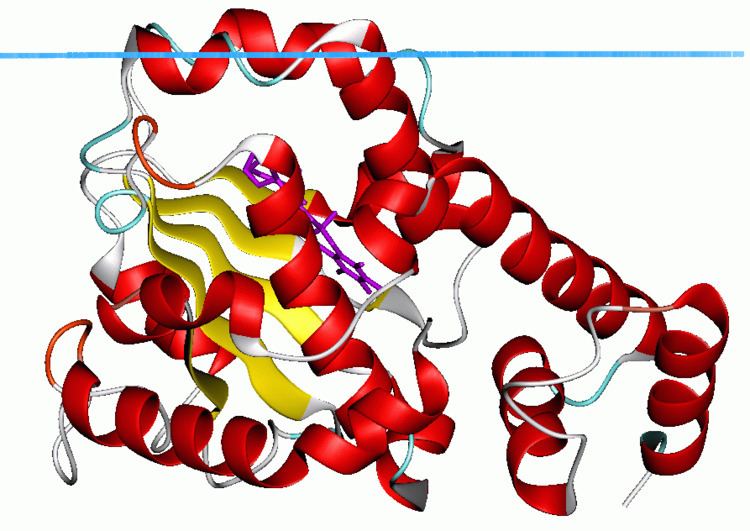
Structure
The Sec14 protein was the first CRAL-TRIO domain for which the structure was determined. The structure contains several alpha helices as well as a beta sheet composed of 6 strands. Strands 2,3,4 and 5 form a parallel beta sheet with strands 1 and 6 being anti-parallel. The structure also identified a hydrophobic binding pocket for lipid binding.
Human proteins containing this domain
C20orf121; MOSPD2; PTPN9; RLBP1; RLBP1L1; RLBP1L2; SEC14L1; SEC14L2; SEC14L3; SEC14L4; TTPA;
