Abbreviations HMDS Appearance colorless liquid | Density 770 kg/m³ | |
 | ||
Bis(trimethylsilyl)amine (also known as hexamethyldisilazane, or HMDS) is an organosilicon compound with the molecular formula [(CH3)3Si]2NH. The molecule is a derivative of ammonia with trimethylsilyl groups in place of two hydrogen atoms. This colorless liquid is a reagent and a precursor to bases that are popular in organic synthesis and organometallic chemistry.
Contents
Preparation and reactions
Bis(trimethylsilyl)amine is prepared by treatment of trimethylsilyl chloride with ammonia:
2 (CH3)3SiCl + 3 NH3 → [(CH3)3Si]2NH + 2 NH4ClThe product is usually handled using air-free techniques since it hydrolyzes slowly in humid air.
Alkali metal bis(trimethylsilyl)amides result from the deprotonation of bis(trimethylsilyl)amine. For example, lithium bis(trimethylsilyl)amide (LiHMDS) is prepared using n-butyllithium:
[(CH3)3Si]2NH + BuLi → [(CH3)3Si]2NLi + C4H10Together with sodium bis(trimethylsilyl)amide (NaHMDS) and potassium bis(trimethylsilyl)amide (KHMDS), LiHMDS is used as a non-nucleophilic base.
Reactions
One of the uses of HMDS is as a reagent in condensation reactions of heterocyclic compounds such as in the microwave synthesis of a derivative of xanthine:
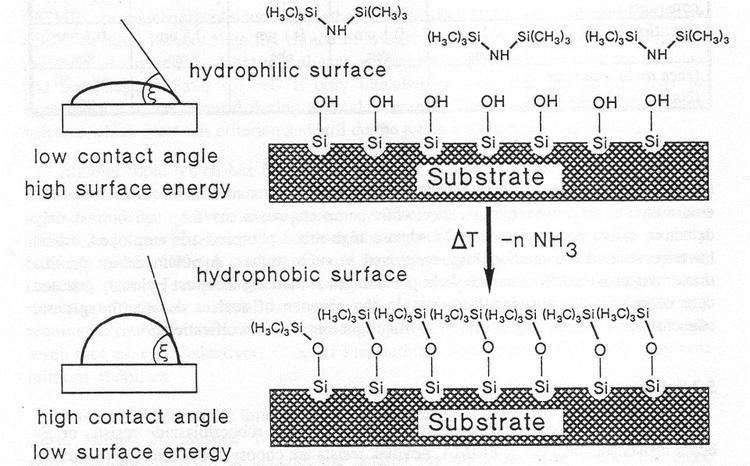
HMDS can be used to convert alcohols into trimethylsilyl ethers. HMDS can be used to silylate laboratory glassware and make it hydrophobic, or automobile glass, just as Rain-X does.
In gas chromatography, HMDS can be used to silylate OH groups of organic compounds to increase volatility, this way enabling GC-analysis of chemicals that are otherwise non-volatile.
Other uses
In photolithography, HMDS is often used as an adhesion promoter for photoresist. Best results are obtained by applying HMDS from the gas phase on heated substrates.
In electron microscopy, HMDS can be used as an alternative to critical point drying during sample preparation.
In pyrolysis-gas chromatography-mass spectrometry, HMDS is added to the analyte to create sylilated diagnostic products during pyrolysis, in order to enhance detectability of compounds with polar functional groups.
