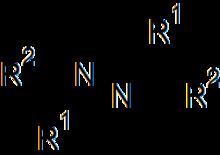 | ||
Fallout 4 all picket fences magazines location guide
Azines are a functional class of organic compounds with the connectivity RR'C=N-N=CRR'. These compounds are the product of the condensation of hydrazine with ketones and aldehydes, although in practice they are often made by alternative routes.
Contents
- Fallout 4 all picket fences magazines location guide
- Fallout 4 u s picket fences comic book magazine locations 5 issues new settlement statues
- Preparation
- Reactions
- Applications
- Nomenclature
- References
Fallout 4 u s picket fences comic book magazine locations 5 issues new settlement statues
Preparation
The usual method of industrial production is the Peroxide process, starting from the ketone, ammonia, and hydrogen peroxide.
In the laboratory, azines are typically prepared by the direct reaction of a carbonyl compound with hydrazine hydrate: the reaction is exothermic.
Reactions
Azines characteristically undergo hydrolysis to hydrazines.
Azines have been used as precursors to hydrazones and diazo compounds.
The coordination chemistry of azines (as ligands) has also been studied. Acetone is used as a derivatize to hydrazine, through formation of acetone azine, for analysis by gas chromatography: the method has been used to determine trace levels of hydrazine in drinking water and pharmaceuticals.
Applications
Ketazines are also important intermediates in the industrial production of hydrazine hydrate by the Peroxide process. In the presence of an oxidant, ammonia and ketones react to give hydrazine via ketazine:
2 Me(Et)C=O + 2 NH3 + H2O2 → Me(Et)C=NN=C(Et)Me + 2 H2OThe ketazine can be hydrolyzed to the hydrazine and regenerate the ketone:
Me(Et)C=NN=C(Et)Me + 2 H2O → 2 Me(Et)C=O + N2H4Ketazines have been also used as sources of hydrazine produced in situ, for example in the production of herbicide precursor 1,2,4-triazole.
Nomenclature
They may be further classified as aldazines or ketazines, depending on the nature of the carbonyl compound.
Azines may also be named by substitutive or functional class nomenclature. In functional class nomenclature, the functional modifier "azine" is appended to the name of the carbonyl compound: hence, "acetone azine". In older nomenclature, the functional class name "ketazine" has been used with the names of the hydrocarbyl substituents: e.g., "methyl ethyl ketazine". In substitutive nomenclature, azines are named as derivatives of hydrazine: hence, "diisopropylidenehydrazine". In the presence of groups of higher seniority, the prefixes "hydrazinylidene" and "hydrazinediylidene" are used.
Unsymmetrical azines, that is compounds of the type X=N–N=Y with X ≠ Y, are not named as azines: in the absence of other functional groups having higher seniority, they can be named as substituted hydrazones.
