Formula AlF3 Molar mass 83.9767 g/mol | Density 2.88 g/cm³ Classification Aluminum compounds | |
 | ||
Appearance white, crystalline solidodorless | ||
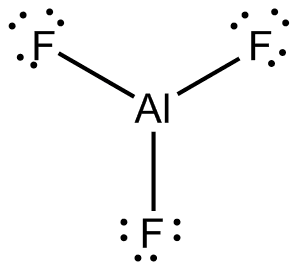
Aluminium fluoride alf3
Aluminium fluoride (AlF3) is an inorganic compound used primarily in the production of aluminium. This colorless solid can be prepared synthetically but also occurs in nature.
Contents

Production and occurrence
The majority of aluminium fluoride is produced by treating alumina with hexafluorosilicic acid:
H2SiF6 + Al2O3 → 2 AlF3 + SiO2 + H2O
Alternatively, it is manufactured by thermal decomposition of ammonium hexafluoroaluminate. For small scale laboratory preparations, AlF3 can also be prepared by treating aluminium hydroxide or aluminium metal with HF.
Aluminium fluoride trihydrate is found in nature as the rare mineral rosenbergite.
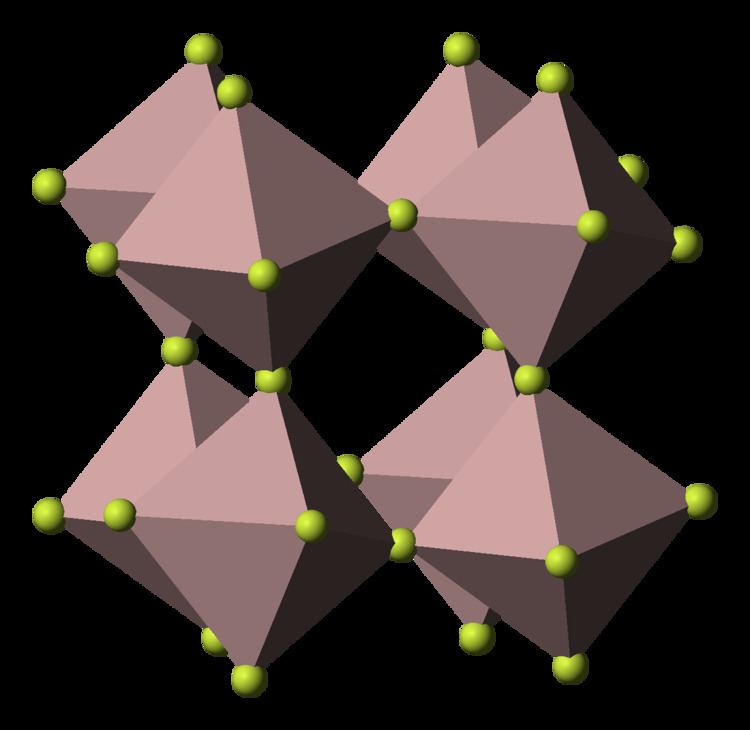
Structure
Its structure adopts the rhenium trioxide motif, featuring distorted AlF6 octahedra. Each fluoride is connected to two Al centers. Because of its 3-dimensional polymeric structure, AlF3 has a high melting point. The other trihalides of aluminium in the solid state differ, AlCl3 has a layer structure and AlBr3 and AlI3, are molecular dimers. Also they have low melting points and evaporate readily to give dimers. In the gas phase aluminium fluoride exists as trigonal molecules of D3h symmetry. The Al-F bond lengths of this gaseous molecule are 163 pm.
Applications
Aluminium fluoride is an important additive for the production of aluminium by electrolysis. Together with cryolite, it lowers the melting point to below 1000 °C and increases the conductivity of the solution. It is into this molten salt that aluminium oxide is dissolved and then electrolyzed to give bulk Al metal.
Niche uses
Together with zirconium fluoride, aluminium fluoride is an ingredient for the production of fluoroaluminate glasses.
It is also used to inhibit fermentation.
Like magnesium fluoride it used as a low-index optical thin film, particularly when far UV transparency is required. Its deposition by physical vapor deposition, particularly by evaporation, is favorable.
Safety
AlF3 has low toxicity (LD50 600 mg/kg).
