Density 1.39 g/cm³ Formula C2H3F3O Appearance Colorless liquid | Boiling point 78 °C Molar mass 100.04 g/mol | |
 | ||
Related compounds | ||
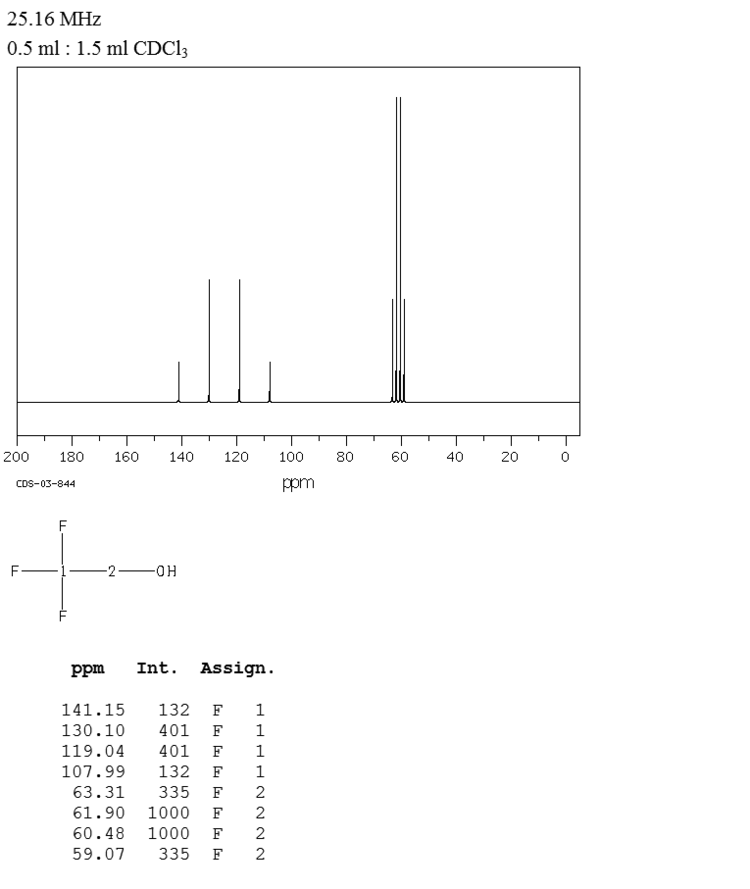
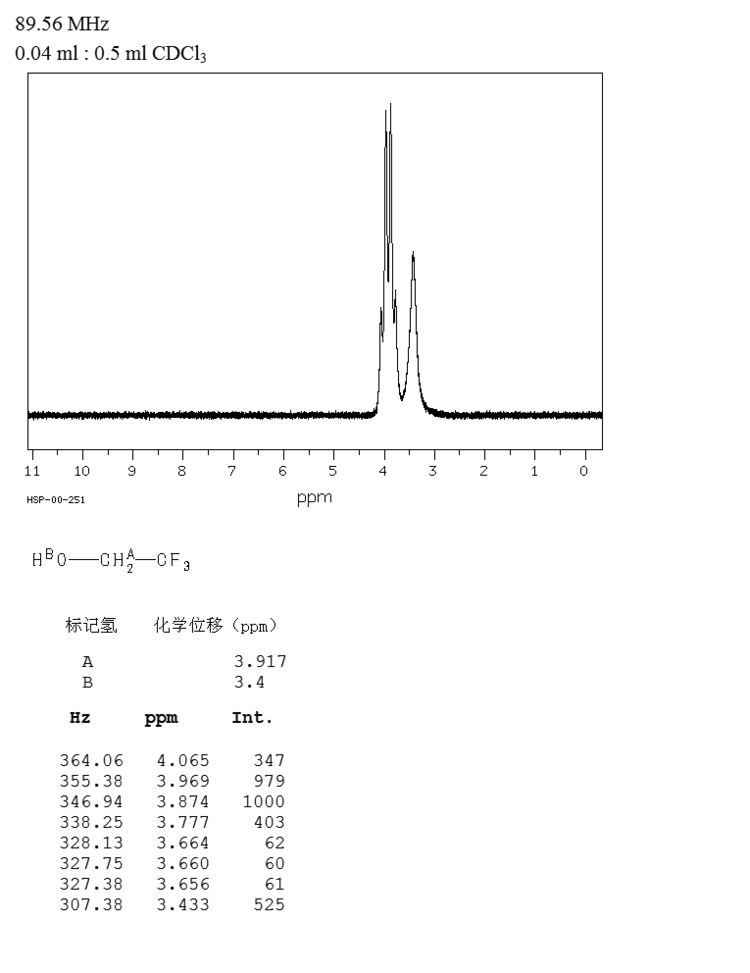
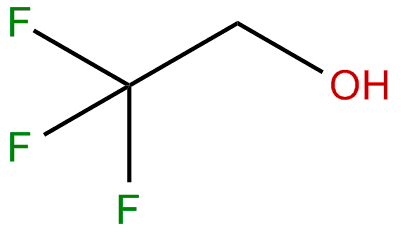
2,2,2-Trifluoroethanol is the organic compound with the formula CF3CH2OH. Also known as TFE or trifluoroethyl alcohol, this colourless, water-miscible liquid has a smell reminiscent of ethanol. Due to the electronegativity of the trifluoromethyl group, this alcohol exhibits a stronger acidic character compared to ethanol. Thus, TFE forms stable complexes also with heterocycles (e.g. THF or pyridine) through hydrogen bonding.
Contents
Synthesis

Trifluoroethanol is produced industrially by hydrogenation or the hydride reduction of derivatives of trifluoroacetic acid, such as the esters or acid chloride.
TFE can also be prepared by hydrogenolysis of compounds of generic formula CF3−CHOH−OR (where R is hydrogen or an alkyl group containing from one to eight carbon atoms), in the presence of a palladium containing catalyst deposited on activated charcoal. As a co-catalyst for this conversion tertiary aliphatic amines like triethylamine are commonly employed.
Uses
Trifluoroethanol is used as a solvent in organic chemistry. Oxidations of sulfur compounds using hydrogen peroxide are effectively conducted in TFE. It can also be used as a protein denaturant. In biology TFE is used as a co-solvent in protein folding studies with NMR spectroscopy: this solvent can effectively solubilize both peptides and proteins. Depending upon its concentration, TFE can strongly affect the three-dimensional structure of proteins.
Industrially trifluoroethanol is employed as a solvent for nylon as well as in applications of the pharmaceutical field.
Reactions
Oxidation of trifluoroethanol yields trifluoroacetaldehyde or trifluoroacetic acid. It also serves as a source of the trifluoromethyl group for various chemical reactions (Still-Gennari modification of HWE reaction).
2,2,2-trifluoro-1-vinyloxyethane, an inhaled drug introduced clinically under the tradename Fluoromar, features a vinylether of trifluorethanol. This species was prepared by the reaction of trifluoroethanol with acetylene.
Safety
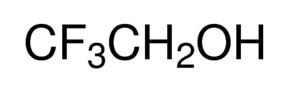
Trifluoroethanol is classified as toxic to blood, the reproductive system, bladder, brain, upper respiratory tract and eyes. Research has shown it to be a testicular toxicant in rats and dogs.
