 | ||
Virtual colony count (VCC) is a kinetic, 96-well microbiological assay originally developed to measure the activity of defensins. It has since been applied to other antimicrobial peptides including LL-37.
Contents
Background
Antimicrobial susceptibility testing (AST) can be done on 96-well plates by diluting the antimicrobial agent at varying concentrations in broth inoculated with bacteria and measuring the minimum inhibitory concentration that results in no growth. However, these methods cannot be used to study some membrane-active antimicrobial peptides, which are inhibited by the broth itself. The virtual colony count procedure takes advantage of this fact by first exposing bacterial cells to the active antimicrobial agent in a low-salt buffer for two hours, then simultaneously inhibiting antimicrobial activity and inducing exponential growth by adding broth. The growth kinetics of surviving cells can then be monitored using a temperature-controlled plate reader.
Quantitative growth kinetics
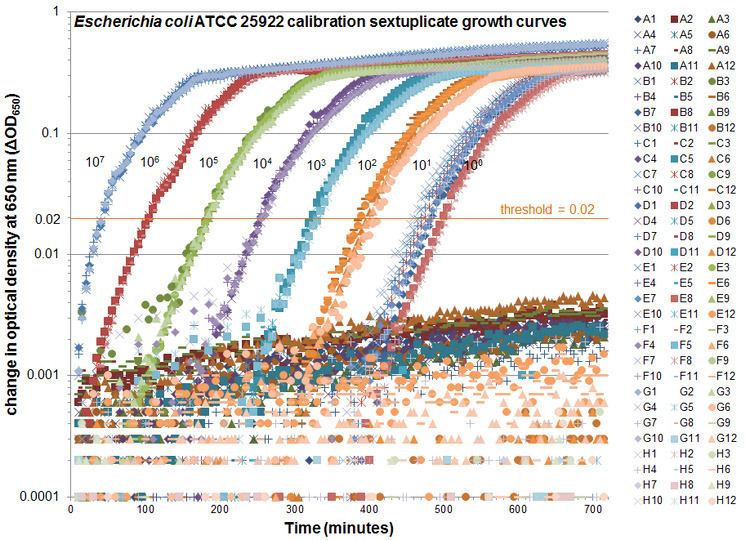
The method of enumeration of surviving cells used by VCC is termed quantitative growth kinetics (QGK). It relates the kinetic time taken for the turbidity of a bacterial batch microbiological culture in a well of a 96-well microplate to reach a threshold difference in turbidity to a 10-fold dilution series of calibration growth curves.
Quantification of the number of viable cells is done using a process mathematically identical to quantitative real-time polymerase chain reaction (QPCR), except with QGK cells, rather than copies of PCR products, grow exponentially. The time taken to reach the threshold is called the "threshold time", Tt, which is equivalent to the QPCR value "cycle time" or Ct.
There are at least five processes that cause delays in threshold times in VCC assays:
1. Adhesion, causing cells to stick to the microplate and possibly form biofilms. Unless these cells happen to be directly in the light path, their growth will not affect optical density readings.
2. Cohesion, causing cells to aggregate into clumps of various sizes instead of a homogeneous suspension of individual cells undergoing binary fission. Cohesion can cause imprecision and fluctuations in Tt. Cohesive clumps may also be adhesive, leading to both imprecision due to cohesion and inaccuracy (increased Tt) due to adhesion.
3. Bacteriostatic activity, causing cells to become unable to enter exponential growth even though they are not killed. Transient bacteriostatic activity can cause lag times, increasing Tt.
4. The metabolic lag phase of bacterial growth. Such a lag phase would be expected to occur in the assay as cells growing slowly or not at all during the initial exposure to antimicrobial peptides in the low-salt buffer are shifted to exponential growth upon addition of twice-concentrated rich media. If this metabolic lag phase increases in the presence of the antimicrobial peptide, it could be considered a form of transient bacteriostatic activity in category 3, above, although other sources of transient bacteriostatic activity, such as a delay due to the time required for the repair of damaged cell structures such as cell walls or cell membranes, are possible.
5. Bactericidal activity, or killing. Fewer surviving cells cause a delay in Tt as the survivors take longer to produce the same amount of turbidity through exponential growth. If all other processes causing increases in Tt are negligible, the VCC assay becomes a bactericidal assay and Tt can be used to enumerate viable bacteria by QGK. In this simplified case, VCC "virtual survival" results are equivalent to the "survival" results of a traditional colony count bactericidal assay.
Bacteria
VCC was initially employed to quantify the antibacterial activity of peptides against six strains of Escherichia coli, Staphylococcus aureus, Bacillus cereus, and Enterobacter aerogenes. Commonly, a standard Gram-negative and Gram-positive quality control strain are compared. Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 29213 have been used as the standard Gram-negative and Gram-positive strains, respectively. VCC has also been applied to Bacillus anthracis, the causative agent of anthrax.
Antimicrobial peptides
The initial virtual colony count study measured the activity of all six human alpha defensins concurrently on the same 96-well plate: HNP1, HNP2, HNP3, HNP4, HD5, and HD6. Subsequently, mutated forms of some of those six defensins were studied by VCC. A conserved glycine in a beta bulge in HNP2 was replaced with a series of D-amino acids resulting in VCC activity proportional to side chain hydrophobicity and charge. VCC showed that N-terminally acetylated and/or C-terminally amidated HNP2 activity is proportional to electrostatic charge. VCC results were again proportional to charge for a series of salt bridge-disrupting mutants, suggesting that the salt bridge is not required for HNP2 function. VCC measured the importance of N-terminal natural and artificial pro segments of the propeptide HNP1, dramatically altering activity against Escherichia coli and Staphylococcus aureus. Enantiomer forms of HNP1, HNP4, HD5 and the beta defensin HBD2 composed entirely of D-amino acids suggested differing mechanisms of defensin activity against Gram-positive and Gram-negative bacteria. VCC results of dimerization-impaired monomer and tethered dimer forms of HNP1 demonstrated the importance of dimerization. Replacing the conserved glycine with L-alanine resulted in subtle VCC differences. Comprehensive alanine scanning mutagenesis of HNP1 and HD5 demonstrated the importance of bulky hydrophobic residues. These studies have recently been expanded to include additional beta defensins, theta defensins, and the human cathelicidin LL-37 and related peptides.
Safe and effective pipetting technique
VCC users are cautioned to transfer cells in a small volume such as 10 microliters beneath a larger volume such as 90 microliters, similar to the QGK calibration curves shown above and the calibration curves reported in the initial VCC publication, but unlike the experimental procedure used to test defensin activity in that same paper. The improved pipetting technique was described in 2011 in the study of the biosafety level 3 (BSL-3) pathogen Bacillus anthracis. The original method published in 2005 involved the transfer of 50 microliters of cell suspensions to 50 microliters of liquid, which generates froth, bubbles and turbidity that is incompatible with a turbidimetric method when cells are transferred directly to the bottoms of the wells beneath the phosphate buffer solutions. Avoiding this problem by adding cell suspensions as droplets from above can cause aerosols that result in cross-contamination. Bioaerosols of hazardous bacteria can also pose safety risks that can be reduced by conducting experiments within a biosafety cabinet.
