 | ||
Upsalite is an anhydrous form of magnesium carbonate first reported in July 2013. With a surface area of 800 m² per gram, Upsalite is reported to have the highest surface area measured for an alkali earth metal carbonate ever created. It is found to adsorb more water at low relative humidities better than the best materials previously available — the hygroscopic zeolites. Further, upsalite can release that water at lower temperatures than zeolites, requiring less energy.
Contents
Naming
The material was given the name Upsalite as a reference to Uppsala University where it was first reported at the lab. There is only one "p" in "upsalite", even though the university's town is spelled "Uppsala". However, "Upsala" is the old spelling of the town, and there is only one "p" in the (Neo-)Latin name of the university, Universitas Regia Upsaliensis.
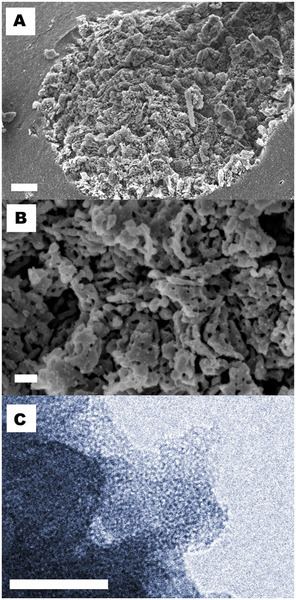
Synthesis
At the laboratory, magnesium oxide (MgO) and methanol are reacted under a carbon dioxide (CO2) pressure of 3 bar at 50 °C for 3 h. Cooling down results in a gel which after depressurization is solidified in a furnace at 70 °C for 2 days. This material can be calcined at 300 °C, which results in pure dry MgCO3 (Upsalite).
Potential uses
Potential uses are the reduction of the amount of energy needed to control environmental moisture in the electronics and drug formulation industry as well as in ice hockey rink and warehouses. It can also be potentially used for collection of toxic waste, chemicals or oil spill and in drug delivery systems, for odor control, sanitation after fire, and the collection of water from any source containing it.
